Abstract
Background
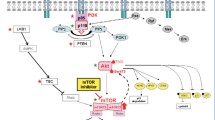
Ovarian cancer is one of the major causes of death in women worldwide. Despite improvements in conventional treatment approaches, such as surgery and chemotherapy, a majority of patients with advanced ovarian cancer experience relapse and eventually succumb to the disease; the outcome of patients remains poor. Hence, new therapeutic strategies are urgently required. The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) is activated in approximately 70 % of ovarian cancers, resulting in hyperactive signaling cascades that relate to cellular growth, proliferation, survival, metabolism, and angiogenesis. Consistent with this, a number of clinical studies are focusing on PI3K pathway as an attractive target in the treatment of ovarian cancer. In this review, we present an overview of PI3K pathway as well as its pathological aberrations reported in ovarian cancer. We also discuss inhibitors of PI3K pathway that are currently under clinical investigations and the challenges these inhibitors face in future clinical utility.
Methods
PubMed was searched for articles of relevance to ovarian cancer and the PI3K pathway. In addition, the ClinicalTrials.gov was also scanned for data on novel therapeutic inhibitors targeting the PI3K pathway.
Results
Genetic aberrations at different levels of PI3K pathway are frequently observed in ovarian cancer, resulting in hyperactivation of this pathway. The alterations of this pathway make the PI3K pathway an attractive therapeutic target in ovarian cancer. Currently, several inhibitors of PI3K pathway, such as PI3K/AKT inhibitors, rapamycin analogs for mTOR inhibition, and dual PI3K/mTOR inhibitors are in clinical testing in patients with ovarian cancer.
Conclusions
PI3K pathway inhibitors have shown great promise in the treatment of ovarian cancer. However, further researches on selection patients that respond to PI3K inhibitors and exploration of effective combinatorial therapies are required to improve the management of ovarian cancer.

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62(1):10–29
Ozols RF (2006) Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol 33(2 Suppl 6):S3–S11
Waldmann A, Eisemann N, Katalinic A (2013) Epidemiology of malignant cervical, corpus uteri and ovarian tumours—current data and epidemiological trends. Geburtsh Frauenheilk 73(2):123–129
Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M et al (2009) Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III trial of the gynecologic cancer intergroup. J Clin Oncol 27(9):1419–1425
Bartholomeusz C, Gonzalez-Angulo AM (2012) Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets 16(1):121–130
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7(8):606–619
Whitman M, Downes CP, Keeler M, Keller T, Cantley L (1988) Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332(6165):644–646
Samuels Y, Ericson K (2006) Oncogenic PI3K and its role in cancer. Curr Opin Oncol 18(1):77–82
Markman B, Atzori F, Perez-Garcia J, Tabernero J, Baselga J (2010) Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol 21(4):683–691
Burman C, Ktistakis NT (2010) Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett 584(7):1302–1312
Backer JM (2008) The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410(1):1–17
Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17:615–675
Vanhaesebroeck B, Waterfield MD (1999) Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 253(1):239–254
Miao B, Skidan I, Yang J, Lugovskoy A, Reibarkh M, Long K et al (2010) Small molecule inhibition of phosphatidylinositol-3,4,5-triphosphate (PIP3) binding to pleckstrin homology domains. Proc Natl Acad Sci USA 107(46):20126–20131
Cantley LC, Neel BG (1999) New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96(8):4240–4245
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273(22):13375–13378
Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D et al (2009) Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16(2):115–125
Staal SP (1987) Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 84(14):5034–5037
Vanhaesebroeck B, Alessi DR (2000) The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346(Pt 3):561–576
Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH (2006) Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal 18(12):2262–2271
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24(50):7443–7454
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R et al (2003) Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res 63(1):196–206
Maroulakou IG, Oemler W, Naber SP, Tsichlis PN (2007) Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res 67(1):167–177
Liby TA, Spyropoulos P, Buff Lindner H, Eldridge J, Beeson C, Hsu T et al (2012) Akt3 controls vascular endothelial growth factor secretion and angiogenesis in ovarian cancer cells. Int J Cancer 130(3):532–543
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9(3):316–323
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Brugge J, Hung MC, Mills GB (2007) A new mutational AKTivation in the PI3K pathway. Cancer Cell 12(2):104–107
Bast RC Jr, Hennessy B, Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9(6):415–428
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4(12):988–1004
Prat J (2004) Pathology of the ovary. Sanders, Philadelphia
Prat J (2012) New insights into ovarian cancer pathology. Ann Oncol 23(Suppl 10):x111–117
Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS et al (2004) Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 64(21):7678–7681
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL et al (2009) Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol 174(5):1597–1601
Kolasa IKRA, Felisiak A, Ziolkowska-Seta I, Murawska M, Moes J, Timorek A, Dansonka-Mieszkowska D, Kupryjanczyk J (2009) PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther 8:21–26
Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS et al (2011) PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther 10(3):558–565
Philp AJCI, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Philips WA (2001) The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res 15(61):7426–7429
Shekar SC, Wu H, Fu Z, Yip SC, Nagajyothi Cahill SM et al (2005) Mechanism of constitutive phosphoinositide 3-kinase activation by oncogenic mutants of the p85 regulatory subunit. J Biol Chem 280(30):27850–27855
Huang CH, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW et al (2007) The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3 K alpha mutations. Science 318(5857):1744–1748
Sansal I, Sellers WR (2004) The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22(14):2954–2963
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M et al (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091
Choucair K, Ejdelman J, Brimo F, Aprikian A, Chevalier S, Lapointe J (2012) PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 12:543
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13(5):283–296
Koshiro Obata SJM, Watson Richard H, Hitchcock Andrew, Chenevix-Trench Georgia, Thomas Eric J, Campbell Ian G (1998) Frequent PTEN/MMAC Mutations in Endometrioid but not Serous or mucinous epithelial ovarian tumors. Cancer Res 58(10):2095–2097
Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM et al (2012) Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol 13(4):385–394
Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T et al (2000) Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res 60(24):7052–7056
Lee Y-K, Park N-H (2009) Prognostic value and clinicopathological significance of p53 and PTEN in epithelial ovarian cancers. Gynecol Oncol 112(3):475–480
Saga Y, Mizukami H, Suzuki M, Kohno T, Urabe M, Ozawa K et al (2002) Overexpression of PTEN increases sensitivity to SN-38, an active metabolite of the topoisomerase I inhibitor irinotecan, in ovarian cancer cells. Clin Cancer Res 8(5):1248–1252
Lou Y, Yang X, Wang F, Cui Z, Huang Y (2010) MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int J Mol Med 26(6):819–827
Fu X, Tian J, Zhang L, Chen Y, Hao Q (2012) Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett 586(9):1279–1286
Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM et al (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448(7152):439–444
Cancer Genome Atlas Research N (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474(7353):609–615
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC et al (1992) AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA 89(19):9267–9271
Kurman RJ, Shih Ie M (2011) Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol 42(7):918–931
Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y et al (2006) Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther 5(7):779–785
Hanrahan AJ, Schultz N, Westfal ML, Sakr RA, Giri DD, Scarperi S et al (2012) Genomic complexity and AKT dependence in serous ovarian cancer. Cancer Discov 2(1):56–67
Balakrishnan A, Chaillet JR (2013) Role of the inositol polyphosphate-4-phosphatase type II Inpp4b in the generation of ovarian teratomas. Dev Biol 373(1):118–129
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269(7):5241–5248
Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C et al (1994) Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res 54(9):2419–2423
Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB (2000) In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin Cancer Res 6(3):880–886
Fekete M, Santiskulvong C, Eng C, Dorigo O (2012) Effect of PI3K/Akt pathway inhibition-mediated G1 arrest on chemosensitization in ovarian cancer cells. Anticancer Res 32(2):445–452
Knight ZA, Shokat KM (2007) Chemically targeting the PI3K family. Biochem Soc Trans 35(Pt 2):245–249
Marone R, Cmiljanovic V, Giese B, Wymann MP (2008) Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 1784(1):159–185
Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D et al (2012) Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 30(3):282–290
ClinicalTrials.gov.Available from: www.clinicaltrials.gov
Juric D, Argiles G, Burris H, Gonzalez-Angulo A, Saura C, Quadt C et al (2012) Phase I study of BYL719, an alpha-specific PI3 K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-positive (ER +) metastatic breast cancer (MBC). Cancer Res 72(24 Supplement):P6–10
Graupera M, Guillermet-Guibert J, Foukas LC, Phng L-K, Cain RJ, Salpekar A et al (2008) Angiogenesis selectively requires the p110 & agr; isoform of PI3K to control endothelial cell migration. Nature 453(7195):662–666
Kang S, Denley A, Vanhaesebroeck B, Vogt PK (2006) Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103(5):1289–1294
Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8(8):627–644
Fu S, Hennessy BT, Ng CS, Ju Z, Coombes KR, Wolf JK et al (2012) Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol 126(1):47–53
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K et al (2010) MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9(7):1956–1967
Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K et al (2011) First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 29(35):4688–4695
Alvarado Y, Mita MM, Vemulapalli S, Mahalingam D, Mita AC (2011) Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target Oncol 6(2):69–94
Kuo CJ, Chung J, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR (1992) Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature 358(6381):70–73
Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS (2009) mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg 6(2):47–55
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) mTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345
Fasolo A, Sessa C (2011) Current and future directions in mammalian target of rapamycin inhibitors development. Expert Opin Investig Drugs 20(3):381–394
Ansell SM, Inwards DJ, Rowland KM Jr, Flynn PJ, Morton RF, Moore DF Jr et al (2008) Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the north central cancer treatment group. Cancer 113(3):508–514
Blakely LJ, Buzdar A, Chang HY, Frye D, Theriault R, Valero V et al (2004) A phase I and pharmacokinetic study of TAS-108 in postmenopausal female patients with locally advanced, locally recurrent inoperable, or progressive metastatic breast cancer. Clin Cancer Res 10(16):5425–5431
Yoon DH, Ryu MH, Park YS, Lee HJ, Lee C, Ryoo BY et al (2012) Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer 106(6):1039–1044
Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S et al (2011) Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol 123(1):19–26
Temkin SM, Yamada SD, Fleming GF (2010) A phase I study of weekly temsirolimus and topotecan in the treatment of advanced and/or recurrent gynecologic malignancies. Gynecol Oncol 117(3):473–476
Vlahovic G, Meadows KL, Uronis HE, Morse MA, Blobe GC, Riedel RF et al (2012) A phase I study of bevacizumab, everolimus and panitumumab in advanced solid tumors. Cancer Chemother Pharmacol 70(1):95–102
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H et al (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65(16):7052–7058
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66(3):1500–1508
Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM et al (2007) A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res 67(17):7960–7965
Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D et al (2011) Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res 17(8):2373–2384
Papadopoulos K, Markman B, Tabernero J, Patnaik A, Heath E, DeCillis A et al (2008) A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of a novel PI3K inhibitor, XL765, administered orally to patients (pts) with advanced solid tumors. J Clin Oncol 26:3510
Garlich JR, De P, Dey N, Su JD, Peng X, Miller A et al (2008) A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res 68(1):206–215
Mahadevan D, Chiorean EG, Harris WB, Von Hoff DD, Stejskal-Barnett A, Qi W et al (2012) Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer 48(18):3319–3327
Elser C, Hirte H, Kaizer L, Mackay H, Bindra S, Tinker L et al (2009) Phase II study of MKC-1 in patients with metastatic or resistant epithelial ovarian cancer or advanced endometrial cancer. J Clin Oncol 27(suppl):5577
Glaysher S, Bolton LM, Johnson P, Atkey N, Dyson M, Torrance C et al (2013) Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer 109(7):1786–1794
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6):320–328
Kinross KM, Brown DV, Kleinschmidt M, Jackson S, Christensen J, Cullinane C et al (2011) In vivo activity of combined PI3K/mTOR and MEK inhibition in a Kras(G12D);Pten deletion mouse model of ovarian cancer. Mol Cancer Ther 10(8):1440–1449
Sheppard KE, Cullinane C, Hannan KM, Wall M, Chan J, Barber F et al (2013) Synergistic inhibition of ovarian cancer cell growth by combining selective PI3K/mTOR and RAS/ERK pathway inhibitors. Eur J Cancer 49(18):3936–3944
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Zeng, J. & Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet 290, 1067–1078 (2014). https://doi.org/10.1007/s00404-014-3377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3377-3