Abstract
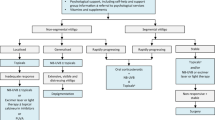
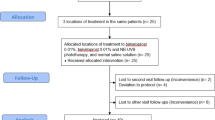
Dexamethasone oral mini-pulse (OMP) is commonly used to halt progression of non-segmental vitiligo (NSV). There is an unmet need for non-phototherapy, non-corticosteroid therapeutic options for stabilizing actively spreading NSV. To assess the efficacy of oral mycophenolate mofetil in stabilizing active NSV in comparison to OMP. In this prospective, randomized, investigator-blinded study, 50 patients of active vitiligo [baseline vitiligo disease activity (VIDA) score 4] were randomized into two groups in 1:1 ratio. Group A received oral dexamethasone (2.5 mg on two successive days a week) and group B received mycophenolate mofetil (up to 2 g) for 180 days with a treatment-free follow-up period of 90 days. Assessment was done using VIDA, number of new lesions in past 30 days, and Vitiligo Area Scoring Index (VASI). Arrest of disease progression was defined as the absence of any new lesions in past 30 days. Twenty-five patients received OMP (group A, 11 males, 14 females), and 25 received mycophenolate (group B, 12 males, 13 females). In both groups, Kruskal–Wallis revealed a significant trend for reduction in VIDA and the number of new lesions in last 30 days, over the treatment and follow-up duration when compared to baseline (p < 0.001). The first significant reduction in VIDA was noticed on 90th day in groups A and B (p < 0.001). In both groups, VIDA reduced significantly at the 180th day compared to baseline (p < 0.001, WMP), only to increase significantly at the 270th day (p < 0.001, WMP). VIDA in group B was marginally higher at 270 days than group A (p 0.03; Mann–Whitney). Eighteen and 17 patients achieved VIDA 2 + on the 180th day in groups A and B, respectively. The mean number of new lesions in last 30 days reduced significantly in both groups at the 180th day (p < 0.001) and 270th day [p < 0.001; Wilcoxon matched pairs (WMP)] when compared to baseline; but increased significantly at the 270th day compared to the 180th day (p 0.006 WMP). Twenty patients in group A and 18 patients in group B had arrest of the disease activity with treatment. Mean duration to arrest disease progression was 47.2 ± 12.1 days in group A, and 52.5 ± 9.3 days in group B; p 0.21. The difference between VASI at baseline and VASI at the 180 and 270th days was non-significant in both groups (p 0.18 WMP). Five patients in each group failed the respective treatments. Acne (n = 3), weight gain (n = 3), headache, insomnia and menstrual irregularity (n = 1 each) were the important adverse effects noted with dexamethasone pulse; whereas nausea (n = 6) and diarrhea (n = 4) were the commonest adverse effects noted with mycophenolate. Two patients in group B discontinued treatment because of leucopenia (n = 1) and transaminitis (n = 1) that resolved after the discontinuation of mycophenolate. Both OMP and mycophenolate mofetil halt actively spreading vitiligo, and have distinct adverse effect profiles. These should be offered in progressive vitiligo, especially in circumstances precluding the use of phototherapy. Relapse occurred significantly earlier with mycophenolate, and relapse rate was higher (though non-significant) than dexamethasone OMP. The repigmentation potential is minimal for both therapies. This study was approved by Institute Ethics Committee, and retrospectively registered with clinical trial registry of India (CTRI/2018/02/011,664).




Similar content being viewed by others
References
Bishnoi A, Parsad D. Clinical and Molecular Aspects of Vitiligo Treatments. Int J Mol Sci. 2018;19.
Pasricha JS, Khaitan BK (1993) Oral mini-pulse therapy with betamethasone in vitiligo patients having extensive or fast-spreading disease. Int J Dermatol 32:753–757
Kanwar AJ, Mahajan R, Parsad D (2013) Low-dose oral mini-pulse dexamethasone therapy in progressive unstable vitiligo. J Cutan Med Surg 17:259–268
Parsad D, Kanwar A (2010) Oral minocycline in the treatment of vitiligo–a preliminary study. Dermatol Ther 23:305–307
Singh A, Kanwar AJ, Parsad D et al (2014) Randomized controlled study to evaluate the effectiveness of dexamethasone oral minipulse therapy versus oral minocycline in patients with active vitiligo vulgaris. Indian J Dermatol Venereol Leprol 80:29–35
Singh H, Kumaran MS, Bains A et al (2015) A Randomized Comparative Study of Oral Corticosteroid Minipulse and Low-Dose Oral Methotrexate in the Treatment of Unstable Vitiligo. Dermatology 231:286–290
Patra S, Khaitan BK, Sharma VK, et al. A randomized comparative study of the effect of betamethasone oral mini-pulse therapy versus oral azathioprine in progressive non-segmental vitiligo. J Am Acad Dermatol. 2019.
Handjani F, Aghaei S, Moezzi I et al (2017) Topical mycophenolate mofetil in the treatment of vitiligo: a pilot study. Dermatol Pract Concept 7:31–33
Njoo MD, Das PK, Bos JD et al (1999) Association of the Kobner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol 135:407–413
Parsad D, Dogra S, Kanwar AJ (2003) Quality of life in patients with vitiligo. Health Qual Life Outcomes 1:58
Gupta V, Sreenivas V, Mehta M et al (2014) Measurement properties of the Vitiligo Impact Scale-22 (VIS-22), a vitiligo-specific quality-of-life instrument. Br J Dermatol 171:1084–1090
Bae JM, Jung HM, Hong BY et al (2017) Phototherapy for Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol 153:666–674
Bhatnagar A, Kanwar AJ, Parsad D et al (2007) Psoralen and ultraviolet A and narrow-band ultraviolet B in inducing stability in vitiligo, assessed by vitiligo disease activity score: an open prospective comparative study. J Eur Acad Dermatol Venereol 21:1381–1385
Allison AC (2005) Mechanisms of action of mycophenolate mofetil. Lupus 14(Suppl 1):s2–8
Richmond JM, Strassner JP, Rashighi M et al (2019) Resident Memory and Recirculating Memory T Cells Cooperate to Maintain Disease in a Mouse Model of Vitiligo. J Invest Dermatol 139:769–778
Izeradjene K, Quemeneur L, Michallet MC et al (2001) Mycophenolate mofetil interferes with interferon gamma production in T-cell activation models. Transplant Proc 33:2110–2111
Majid I, Masood Q, Hassan I et al (2009) Childhood vitiligo: response to methylprednisolone oral minipulse therapy and topical fluticasone combination. Indian J Dermatol 54:124–127
Eleftheriadou V, Thomas KS, Whitton ME et al (2012) Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol 167:804–814
Imamura S, Tagami H (1976) Treatment of vitiligo with oral corticosteroids. Dermatologica 153:179–185
Radakovic-Fijan S, Furnsinn-Friedl AM, Honigsmann H et al (2001) Oral dexamethasone pulse treatment for vitiligo. J Am Acad Dermatol 44:814–817
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
None.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bishnoi, A., Vinay, K., Kumaran, M.S. et al. Oral mycophenolate mofetil as a stabilizing treatment for progressive non-segmental vitiligo: results from a prospective, randomized, investigator-blinded pilot study. Arch Dermatol Res 313, 357–365 (2021). https://doi.org/10.1007/s00403-020-02108-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-020-02108-8