Abstract
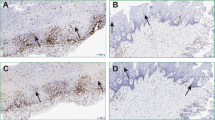
Oral lichen planus (OLP) is a complex immunological disorder, mediated in part by the release of cytokines by activated T-cells. Recently, the role of novel cytokines including IL33 and IL35 has been described in various chronic inflammatory diseases. IL33, a member of the IL-1 superfamily of cytokines, functions as an ‘alarmin’ released after cell necrosis to alert the immune system to tissue damage or stress. IL35, a member of IL12 cytokine family, is produced by regulatory T-cells and suppresses the immune response. The expression of IL33 and IL35 is yet to be investigated in OLP. The aim of this study was to determine the presence and topographical distribution of IL33 and IL35 in OLP using immunohistochemistry and quantitative real-time reverse transcriptase polymerase chain reaction (qPCR). For IHC, formalin-fixed paraffin-embedded archival specimens of OLP (n = 10) and a non-specific inflammatory (NSI) control group (n = 9) were used. A double-labelling immunofluorescence technique was used to determine the expression of IL33 and IL35 on CD3+ T-cells. In addition, 12 fresh tissue samples (OLP n = 6 and NSI controls n = 6) were used to determine the gene expression of IL33 and EBI3 (one chain of the dimeric IL35). Quantitative and qualitative analysis was performed with statistical significance set at p < 0.05. IHC showed positive immunostaining with IL33 and IL35 in both OLP and NSI. Comparison of the numbers of IL33+ and IL35+ cells in OLP and NSI did not show any significant difference. In OLP, there were significantly more IL33+ cells in the deeper connective tissue region than at the epithelial–connective tissue interface. Interestingly, all IL35+ cells observed in both OLP and NSI tissues showed ovoid/plasmacytoid morphology. Double-labelling immunofluorescence showed that IL33 and IL35 expression was not localized within CD3+ T-cells. The gene expression experiments showed significantly higher expression of EBI3 (fold regulation 14.02) in OLP when compared to the inflammatory controls. IL33 gene expression was not different between the groups. However, within the OLP tissues, there was a significantly higher expression of IL33 than EBI3. Our data demonstrate the expression of IL33 and IL35 in OLP lesions. Further studies are needed to understand the functional role of these cytokines in OLP pathogenesis.






Similar content being viewed by others
References
Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA et al (2007) Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:e1-12
Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K (2005) Current controversies in oral lichen planus: report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 100:40–51
Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A et al (2002) The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med 13:350–365
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK et al (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490
Allakhverdi Z, Smith DE, Comeau MR, Delespesse G (2007) Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 179:2051–2054
Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A et al (2012) IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol 21:876–894
Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S et al (2009) Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheumatol 60:738–749
Borthwick LA (2016) The IL-2 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol 2016 38:517–534
Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C et al (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31:84–98
Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE (2008) IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 20(8):1019–1030
Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L et al (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Nat Acad Sci USA 104:282–287
Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M (2012) Regulation of IL-33 expression by IFN-gamma and tumor necrosis factor-alpha in normal human epidermal keratinocytes. J Investig Dermatol 132:2593–2600
Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM et al (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450:566–569
Devergne O, Birkenbach M, Kieff E (1997) Epstein–Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Nat Acad Sci USA 94:12041–12046
Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D et al (2012) The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 13:290–299
Pylayeva-Gupta Y (2016) Molecular pathways: interleukin-35 in autoimmunity and cancer. Clin Cancer Res 22:4973–4978
Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA (2011) Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol 186:6661–6666
Dixon KO, van der Kooij SW, Vignali DA, van Kooten C (2015) Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur J Immunol 45:1736–1747
Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E et al (2014) IL-35 producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507:366–370
Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB et al (2007) IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37:3021–3029
Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J et al (2010) IL-35 mediated induction of a potent regulatory T cell population. Nat Immunol 11:1093–1101
van der Meij EH, van der Waal I (2003) Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 32:507–512
van der Waal I (2009) Oral lichen planus and oral lichenoid lesions; a critical appraisal with emphasis on the diagnostic aspects. Med Oral Patol Oral Cir Bucal 14:E310-3144
Zhou Y, Jiang L, Liu J, Zeng X, Chen QM (2010) The prevalence of hepatitis C virus infection in oral lichen planus in an ethnic Chinese cohort of 232 patients. Int J Oral Sci 2:90–97
Javvadi LR, Parachuru VPB, Milne TJ, Seymour GJ, Rich AM (2016) Regulatory T-cells and IL17A+cells infiltrate oral lichen planus lesions. Pathol 48:564–573
Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ (2010) Expression and regulation of interleukin-33 in human monocytes. Immunol 130:172–180
Shimosato T, Fujimoto M, Tohno M, Sato T, Tateo M, Otani H et al (2010) CpG oligodeoxynucleotides induce strong up-regulation of interleukin 33 via Toll-like receptor 9. Biochem Biophys Res Commun 394:81–86
Khan A, Farah CS, Savage NW, Walsh LJ, Harbrow DJ, Sugerman PB (2003) Th1 cytokines in oral lichen planus. J Oral Pathol Med 32:77–83
Simark Mattsson C, Jontell M, Bergenholtz G, Heyden M, Dahlgren UI (1998) Distribution of interferon-gamma mRNA-positive cells in oral lichen planus lesions. J Oral Pathol Med 27:483–488
Simark-Mattsson C, Bergenholtz G, Jontell M, Eklund C, Seymour GJ, Sugerman PB et al (1999) Distribution of interleukin-2, -4, -10, tumour necrosis factor-alpha and transforming growth factor-beta mRNAs in oral lichen planus. Arch Oral Biol 44:499–507
Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F et al (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163:69–79
Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML et al (2011) The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J Immunol 187:1609–1616
Firth FA, Friedlander LT, Parachuru VPB, Kardos TB, Seymour GJ, Rich AM (2015) Regulation of immune cells in oral lichen planus. Arch Dermatol Res 307:333–339
Tao XA, Xia J, Chen XB, Wang H, Dai YH, Rhodus NL et al (2010) FOXP3 T regulatory cells in lesions of oral lichen planus correlated with disease activity. Oral Dis 16:76–82
Manzoor F, Johnson MC, Li C, Samulski RJ, Wang B, Tisch R (2017) β-cell-specific IL-35 therapy suppresses ongoing antoimmune diabetes in NOD mice. Eur J Immunol 47:144–154
Funding
The project was funded by a New Zealand Dental Association Research Foundation grant and this assistance is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have any conflicts of interest with regard to this manuscript.
Ethical approval
The project received ethical approval from the University of Otago Human Ethics Committee (Reference number H13/052) and all patients whose tissue was used had consented to the use their residual tissue, after diagnostic investigations had been completed, for research purposes.
Rights and permissions
About this article
Cite this article
Javvadi, L.R., Parachuru, V.P.B., Milne, T.J. et al. Expression of IL33 and IL35 in oral lichen planus. Arch Dermatol Res 310, 431–441 (2018). https://doi.org/10.1007/s00403-018-1829-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-018-1829-5