Abstract
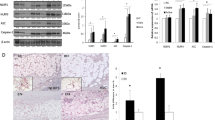
Rosacea is a chronic inflammatory condition with predominant facial involvement. Because of that, many patients sense that rosacea affects quality of life. The etiology of rosacea remains unknown. Recent studies have suggested that aberrant innate immunity is central to this disease. The aim of this study was to examine the presence of Langerhans cells, plasmacytoid dentritic cells (PDC), the expression of Toll-like receptors (TLR) and inducible oxide nitric synthase (iNOS) in skin of patients with rosacea, to highlight the participation of innate immunity in its pathogenesis. 28 biopsy specimens were taken from patients with clinical and histopathological findings of rosacea. Immunohistochemical demonstration of Langerhans cells (anti-CD1a antibody), PDC (anti-CD 123 antibody), TLR2, TLR4 and iNOS was performed in skin samples and compared with normal skin controls. The expression of Langerhans cells was lower in rosacea group than in control group. PDC were found in skin samples of rosacea as isolated cells and forming small clusters. Expression of TLR2, TLR4 and iNOS was higher in rosacea samples than in normal skin controls. This research demonstrates early and late stage components of innate immunity in specimens of rosacea ratifying the existence of an altered innate immunity in its pathogenesis.



Similar content being viewed by others
References
Agnoletti AF, DE Col E, Parodi A, Schiavetti I, Savarino V, Rebora A et al (2016) Etiopathogenesis of rosacea: a prospective study with a three-year follow-up. G Ital Dermatol Venereol 152(5):418–423
Aksoy B, Altaykan-Hapa A, Egemen D, Karagoz F, Atakan N (2010) The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. Br J Dermatol 163:719–725
Bae YI, Yun S, Lee J, Kim S, Won YH, Lee S (2009) Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol 21(3):243–249
Barco D, Alomar A (2008) Rosacea. Actas Dermosifiliogr 99:244–256
Bene MC, Feuillard J, Jacob MC (2003) Plasmacytoid dendritic cells: from the plasmacytoid T-cell to 373 type 2 dendritic cells CD4+ CD56+ malignancies. Semin Hematol 40:257–266
Bieber T, Ring J, Braun-Falco O (1988) Comparison of different methods for enumeration of Langerhans cells in vertical cryosections of human skin. Br J Dermatol 118:385–392
Bogdan C, Rollinghoff M, Diefenbach A (1998) The role of nitric oxide in innate immunity. Immunol Rev 173:17–26
Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V (1998) Nitric oxide in human skin: current status and future prospects. J Invest Dermatol 110:1–7
Cabibi D, Aragona F, Guarnotta C, Rodolico V, Zerilli M, Belmonte B et al (2011) Glut-1 expression and in situ CD1a/CD57 immunologic deficit in keratoacanthoma and squamous cell carcinoma of immunocompetent patients. Appl Immunohistochem Mol Morphol 19(3):239–245
Chan ED, Riches DW (2001) IFN-gamma LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (MAPK) in a mouse macrophage cell line. Am J Physiol Cell Physiol 280:C441–C450
Culp B, Scheinfeld N (2009) Rosacea: a review. PT 34(1):38–45
Diaz C, O’Callaghan CJ, Khan A, Ilchyshyn A (2003) Rosacea: a cutaneous marker of Helicobacter pylori infection? Results of a pilot study. Acta Derm Venereol 83(4):282–286
Elewski BE, Draelos Z, Dreno B, Jansen T, Layton A, Picardo M (2011) Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol 25(2):188–200
Elston DM (2010) Demodex mites: facts and controversies. Clin Dermatol 28(5):502–504
Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL (2001) Plasmacytoid dendritic cells (natural interferon α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 159:237–243
Gravina A, Federico A, Ruocco E, Lo Schiavo A, Masarone M, Tuccillo C et al (2015) Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosácea. United Eur Gastroenterol 3(1):17–24
Hernando-Harder AC, Booken N, Goerdt S, Singer MV, Harder H (2009) Helicobacter pylori infection and dermatologic diseases. Eur J Dermatol 19(5):431–444
Kang SSW, Kauls LS, Gaspari AA (2006) Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol 54:951–983
Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K (2001) Type 1 Helicobacter pylori lipopolysaccharide stimulates Toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun 69:4382–4389
Koreck A, Pivarcsi A, Dobozy A, Kemény L (2003) The role of innate immunity in the pathogenesis of acne. Dermatology 206:96–105
Lacey N, Delaney S, Kavanagh K, Powell FC (2007) Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol 157(3):474–481
Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B et al (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449:564–569
Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R et al (2005) Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci 102(52):19057–19062
Manicassamy S, Pulendran B (2009) Modulation of adaptive immunity with Toll-like receptors. Semin Immunol 21(4):185–193
McNiff JM, Kaplan DH (2008) Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J Cutan Pathol 35:452–456
Merad M, Ginhoux F, Collin M (2008) Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 8(12):935–947
Mizumoto N, Takashima A (2004) CD1a and langerin: acting as more than Langerhans cell markers. J Clin Invest 113:658–660
Murphy KP (2012) An Introduction to immunobiology and innate immunity. In: Janeway’s immunobiology, 8th edn. Garland Science, New York, pp 1–125
Nandi J, Saud B, Zinkievich JM, Yang ZJ, Levine RA (2010) TNF-alpha modulates iNOS expression in an experimental rat model of indomethacin-induced jejunoileitis. Mol Cell Biochem 336(1–2):17–24
Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O et al (2005) Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med 202:135–143
Pagliari C, Sotto MN (2003) Dendritic cells and pattern of cytokines in paracoccidioidomycosis skin lesions. Am J Dermatopathol 25(2):107–112
Robak E, Smolewski P, Wozniacka A, Sysa-Jedrzejowska A, Robak T (2004) Clinical significance of circulating dendritic cells in patients with systemic lupus erythematosus. Mediat Inflamm 13:171–180
Robledo MA, Orduz M (2015) Hypothesis of demodicidosis rosacea flushing etiopathogenesis. Med Hypotheses 84(4):408–412
Rojo-García JM, Muñoz-Pérez MA, Escudero J, Camacho F et al (2000) Helicobacter pylori in rosacea and chronic urticaria. Acta Derm Venereol 80(2):156–157
Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG (2003) TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19(1):59–70
Simões Quaresma JA, de Oliveira MF, Ribeiro Guimarães AC, de Brito EB, de Brito RB, Pagliari C et al (2009) CD1a and factor XIIIa immunohistochemistry in leprosy: a possible role of dendritic cells in the pathogenesis of Mycobacterium leprae infection. Am J Dermatopathol 31(6):527–531
Tüzün Y, Keskin S, Kote E (2010) The role of Helicobacter pylori infection in skin diseases: facts and controversies. Clin Dermatol 28(5):478–482
Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H (2007) Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 293:G1004–G1012
Vermi W, Lonardi S, Morassi M, Rossini C, Tardanico R, Venturini M et al (2009) Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology 214:877–886
Wackernage A, Massone C, Hoefler G, Steinbauer E, Kerl H, Wolf P (2007) Plasmacytoid dendritic cells are absent in skin lesions of polymorphic light eruption. Photodermatol Photoimmunol Photomed 23:24–28
Weller R (2003) Nitric oxide: a key mediator in cutaneous physiology. Clin Exp Dermatol 28:511–514
Wollenberg A, Wagner M, Gunther S, Towarowski A, Tuma E, Moderer M et al (2002) Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 119:1096–1102
Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A et al (2007) Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 13:975–980
Yamasaki K, Gallo RL (2009) The molecular pathology of rosacea. J Dermatol Sci 55:77–81
Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T et al (2011) TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest dermatol 131:688–697
Zhao YE, Wu LP, Peng Y, Cheng H (2010) Retrospective analysis of the association between demodex infestation and rosacea. Arch Dermatol 146(8):896–902
Funding
Supported by Brazilian financial Grant from CAPES-PROAP (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research ethics committee of the University of São Paulo Medical School approved the study protocol (approval number: 0122/09), that is attached as one of the files in this submission.
Informed consent
Informed consent was obtained from all individual participants included in the study
Rights and permissions
About this article
Cite this article
Moura, A.K.A., Guedes, F., Rivitti-Machado, M.C. et al. Inate immunity in rosacea. Langerhans cells, plasmacytoid dentritic cells, Toll-like receptors and inducible oxide nitric synthase (iNOS) expression in skin specimens: case-control study. Arch Dermatol Res 310, 139–146 (2018). https://doi.org/10.1007/s00403-018-1806-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-018-1806-z