Abstract
Deficiency of dietary choline, an essential nutrient, is observed worldwide, with ~ 90% of Americans being deficient. Previous work highlights a relationship between decreased choline intake and an increased risk for cognitive decline and Alzheimer’s disease (AD). The associations between blood circulating choline and the pathological progression in both mild cognitive impairment (MCI) and AD remain unknown. Here, we examined these associations in a cohort of patients with MCI with presence of either sparse or high neuritic plaque density and Braak stage and a second cohort with either moderate AD (moderate to frequent neuritic plaques, Braak stage = IV) or severe AD (frequent neuritic plaques, Braak stage = VI), compared to age-matched controls. Metabolomic analysis was performed on serum from the AD cohort. We then assessed the effects of dietary choline deficiency (Ch−) in 3xTg-AD mice and choline supplementation (Ch+) in APP/PS1 mice, two rodent models of AD. The levels of circulating choline were reduced while pro-inflammatory cytokine TNFα was elevated in serum of both MCI sparse and high pathology cases. Reduced choline and elevated TNFα correlated with higher neuritic plaque density and Braak stage. In AD patients, we found reductions in choline, its derivative acetylcholine (ACh), and elevated TNFα. Choline and ACh levels were negatively correlated with neuritic plaque load, Braak stage, and TNFα, but positively correlated with MMSE, and brain weight. Metabolites L-Valine, 4-Hydroxyphenylpyruvic, Methylmalonic, and Ferulic acids were significantly associated with circuiting choline levels. In 3xTg-AD mice, the Ch− diet increased amyloid-β levels and tau phosphorylation in cortical tissue, and TNFα in both blood and cortical tissue, paralleling the severe human-AD profile. Conversely, the Ch+ diet increased choline and ACh while reducing amyloid-β and TNFα levels in brains of APP/PS1 mice. Collectively, low circulating choline is associated with AD-neuropathological progression, illustrating the importance of adequate dietary choline intake to offset disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that currently affects 6.5 million people aged 65 and older, with an estimated increase to 13.8 million by 2060 [3]. AD is characterized by the presence of amyloid beta (Aβ) plaques and neurofibrillary tau tangles (NFT) that result in progressive loss of memory and other cognitive abilities [52]. While clinical symptomologies of AD typically appear later in life, there is a long preclinical phase which includes molecular dysregulation—e.g., elevations of neuroinflammatory factors such as tumor necrosis factor alpha (TNFα)—which contributes to pathogenesis [14]. A better understanding of preclinical processes could prevent or delay disease development.
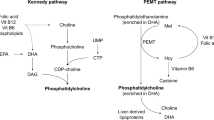
Environmental factors such as a lack of dietary nutirents may contribute to AD pathogenesis. Choline, a B-like micronutrient, plays key roles across a wide range of organ systems, including being a precursor of both acetylcholine (ACh) and cell-membrane lipids [51]. Only 30% of the required choline is produced endogenously by phosphatidylethanolamine-N-methyltransferase (PEMT) in the liver; the rest must be consumed [20]. Dietary guidelines by the Institute of Medicine are focused on liver health and recommend 550- and 425-mg/day for men and women, respectively, and 550 mg/day for pregnant women given fetal developmental requirements [69]. Normal circulating choline concentration in fasted, non-pregnant humans are ~ 9.56 ± 0.49 μmol/L [51]. Circulating choline levels are controlled by choline supply and the ability of tissues to accumulate choline [40]. The rate of choline transport across the blood brain barrier is positively dependent on circulating choline concentration and requires choline transporters [40]. Dietary choline deficiency produces detrimental outcomes including nonalcoholic fatty liver disease, glucose metabolism impairments, cardiovascular disease, and impaired cognition [11, 20]. Alarmingly, choline deficiency is observed worldwide, including ~ 90% of Americans, with average dietary intake of males being 402 mg/day, while females only consume 278 mg/day [60, 69]. This is notable as choline modulates metabolic functions, the dysregulation of which increase AD prevalence [16], and AD incidence is higher in females [52].
Recent work highlights choline’s importance for healthy cognitive aging and that deficiency can contribute to AD development [38, 67, 68]. Older adults who consume between 187.6–399.5 mg/day of choline have less cognitive decline than those consuming < 187.6 mg/day [38]. Additionally, a recent report showed that low dietary choline intake (< 215 mg/day) across the lifespan increases the risk of dementia [68]. However, these reports are based on dietary supplementation questionnaire estimates, and not on measured circulating choline. Further, the rs7946 PEMT single nucleotide polymorphism increases AD incidence [8] and reduction in ACh is a well-documented disease feature of AD [18, 33, 55]. AD mouse models corroborate the importance of dietary choline for healthy aging; dietary choline above the recommended daily amount protects against cognitive decline and AD pathology [58], while choline deficiency exacerbates pathology [11]. Studies assessing circulating choline in AD patients via serum, plasma, or CSF has been mixed; while some studies report that circulating choline decreases in AD cases [13, 22, 56], others show an increase [17, 22, 46] or no change [33, 55]. However, studies to date have not directly measured how differences in circulating choline correspond to specific pathological burdens. Moreover, metabolomic studies show that a plethora of metabolic changes accompany neurological disorders [31, 46]. However, metabolites dysregulated in AD that correspond with alterations in choline have not been elucidated and may reveal novel disease mechanisms and biomarkers of disease progression.
Here, we sought to understand the association between circulating choline in blood and the pathological progression of AD, using samples from patients with mild cognitive impairment (MCI), moderate AD, severe AD, and respective healthy aged-matched controls. Additionally, we examined the role of dietary choline deficiency and supplementation in two mouse models of AD, to understand how the consequences of diet modulation in vivo overlap with differences observed in human patient serum. Lastly, we performed a metabolomic analysis to evaluate disruptions in the human metabolome that are associated with choline and disease progression. We hypothesized that lower circulating choline levels would be associated with increased AD pathologies, while choline supplementation will reduce neuropathology.
Materials and methods
Human serum samples
Human serum samples were obtained from the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program [5, 11]. Pathological assessment of human cases was performed as previously described [6, 12]. Briefly, sections were stained with Campbell-Switzer, Gallyas, and Thioflavin S for plaques, tangles, and other inclusions. Aβ plaque and NFT density were graded and staged at standard sites in frontal, temporal, parietal, and occipital cortices as well are hippocampus and entorhinal cortex. Total plaque score was derived from Campbell-Switzer stain. Neuritic plaque densities were assessed from the Gallyas and Thioflavin S stains. All three stains show neurofibrillary changes, and the score was taken from a combined assessment of all three stains. These are the stains originally used by Braak and Thal to develop and publish Braak neurofibrillary stages and Thal amyloid phases. For MCI subject analysis, patients were either healthy controls (n = 10), or diagnosed with MCI with sparse pathology (MCI Sparse; sparse CERAD neuritic plaque density and Braak stage = II–III; n = 10), or MCI with high pathology (MCI High; frequent CERAD neuritic plaque density and Braak stage = IV–V; n = 12; Supplementary Table 1). MCI cases did not meet the criteria for an AD diagnosis. For AD subject analysis, thirty-six samples, balanced for sex, were obtained including healthy controls with Braak stage ≤ III (CON, n = 12), moderate AD with Braak stage = IV and moderate to frequent CERAD neuritic plaque density (AD Mod, n = 12), and severe AD with Braak stage = VI and frequent CERAD neuritic plaque density (AD Sev, n = 12; Supplementary Table 2). AD Mod and AD Sev corresponded with NIA-RI Intermediate and High classifications, respectively [12]. The medical profiles of the subjects were extensively documented both pre- and post-mortem. Profiles included body mass index (BMI), diagnosis age, years since diagnosis, expired age, post-mortem interval (PMI) at autopsy, final Mini-Mental State Examination (MMSE), Global cognitive score, APOE status, Braak stage, CERAD neuritic plaque density, TDP-43 pathology, brain weight, and National Institute on Aging-Regan Institute (NIA-RI) diagnosis. Some cases were prescribed Rivastigmine (n = 1 MCI High), Donepezil (n = 1 MCI High, n = 4 AD Mod, n = 4 AD Sev), and Galantamine (n = 1 AD Sev). One AD Mod case was prescribed both Donepezil and Galantamine. Missing metrics for some cases are denoted in Supplementary Table 1 (MCI) and 2 (AD). TDP-43 burden was assessed in amygdala, hippocampal CA1, dentate granular cell layer, entorhinal/transentorhinal area, middle temporal gyrus and middle frontal gyrus via chromogenic immunohistochemistry with an antibody against phosphorylated TDP-43 peptide [4]. Some subjects had TDP-43 proteinopathy but did not meet diagnostic criteria for frontotemporal lobar degeneration with TDP-43. Some subjects met the criteria for cerebral amyloid angiopathy (CAA) and cerebral white matter rarefaction (CWMR). No other neuropathologies were present.
Animals
Mice were kept on a 12-h light/dark cycle at 23 °C with ad libitum access to food and water and group-housed, 4–5 per cage. All animal procedures were approved in advance by the Institutional Animal Care and Use Committee of Arizona State University. 3xTg–AD mice express a Swedish mutation of the amyloid precursor protein (APP), a mutated presenilin 1 (PS1 M146V) to accelerate amyloid deposition, and a mutated human tau (P301L), leading to tau pathology, as previously described [11, 12, 59]. C57BL6/129Svj mice were used as NonTg controls. Consistent with previous literature highlighting inconsistent pathology in male 3xTg-AD, only female mice were used [11, 63]. 3xTg-AD mice develop Aβ starting at 6 months and pathological tau at 12 months [11, 12, 63]. Notably, genetic drift is seen in the 3xTg-AD model [32], and protocols recommended by Jackson Labs to minimize this drift were implemented in this colony. 3xTg-AD and NonTg mice were randomly assigned to one of two diets at 3 months of age; a standard laboratory AIN76A diet (Envigo Teklad Diets, Madison WI) with adequate choline (ChN; 2.0 g/kg choline chloride; #TD.180228), or a AIN76A choline-deficient (Ch−; 0.0 g/kg; #TD.110617) diet, as previously described[11].
APP/PS1 mice were generated for the choline supplementation experiment as previously described [58]. The APP/PS1 mice are hemizygous for the amyloid precursor protein (APP) Swedish mutations (KM670/671Nl) and PS1 deltaE9 mutation. APP/PS1 mice were backcrossed for 12 generations into a pure 129/SvJ background [58]. APP/PS1 mice develop Aβ42 plaques starting at 6 months, with extensive pathology through the hippocampus and cortex at 12 months [29, 42, 58]. At 2.5 months of age, female APP/PS1 and wild‐type (NonTg) mice were randomly assigned to receive one of two concentrations of choline chloride in their diet (Harlan laboratories). The control diet (Ctl; 1.1 g/kg choline chloride) supplies adequate choline and is comparable to amounts in previous studies [5, 57, 61], while the supplemented diet (Ch + ; 5.0 g/kg choline chloride) contains approximately 4.5 times the amount of choline consumed in the Ctl group, allowing assessment of the effects of supplementation.
Mouse tissue and plasma collection
At 7 and 12 months of age, 3xTg-AD and NonTg mice were fasted for 16 h, and 150–200 μL (≤ 1% of the subject’s body weight) of blood was collected via the submandibular vein and placed into EDTA-lined tubes (BD K2EDTA #365,974). Tubes were inverted eight times to assure anticoagulation, kept on ice for 60–90 min, and then centrifuged at 2000 to 3000 g for 30 min at 4 °C to separate phases. The top layer was collected and frozen at − 80 °C prior to use. Plasma samples were used to assess choline, acetylcholine (ACh), and TNFα. 3xTg-AD and NonTg mice were euthanized at a mean age of 12.7 months via perfusion with 1 × Phosphate buffer saline (PBS) or CO2 inhalation. Cortex tissue was dissected, homogenized to extract soluble and insoluble fractions, and stored at − 80 °C, as previously described [11]. Hippocampal tissue for these mice was utilized in a previously published article examining dietary choline deficiency effects [11]. APP/PS1 and NonTg counterparts were euthanized at 12.5 months of age via perfusion with 1 × PBS or CO2 inhalation. The olfactory bulbs and cerebellum were removed, and the remaining brain hemisphere, including cortex, hippocampus, and other midbrain regions, such as striatum, basal forebrain, and brain stem, were collected to assess the widespread effects of choline supplementation. Tissue was homogenized to extract soluble and insoluble fractions, and stored at – 80 °C, as previously described [58].
ELISAs and colorimetric assays
We used commercially available ELISA kits (Invitrogen-ThermoFisher Scientific) to quantify levels of soluble and insoluble Aβ40 and Aβ42, and levels of phosphorylated Tau (pTau) at threonine (Thr) 181 and serine (S) 396, and TNFα (Abcam, ab208348) as previously described [11, 64]. Choline and acetylcholine (ACh) levels in serum, plasma and cortex were quantified using commercially available colorimetric assay kits (Abcam, ab219944; Cell Biolabs, Inc., STA-603). The ACh assay raw value is a measure of total choline—both the choline bound in ACh and free choline. Consequently, the value for ACh that was analyzed was derived from the formula \(\mathrm{Aceytlcholine}=\mathrm{Total Choline}-\mathrm{Free Choline}\), as described by the manufacturer.
Metabolomics analysis
Analytical methanol (MeOH) was purchased from Fisher Scientific (Waltham, MA). Methyl tert-butyl ether (MTBE), O-methyl hydroxylamine hydrochloride (MeOX), N-Methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (MTBSTFA), and pyridine were purchased from Sigma-Aldrich (Saint Louis, MO). Deionized water was provided in-house by a water purification system from EMD Millipore (Billerica, MA). PBS was purchased from GE Healthcare Life Sciences (Logan, UT). Compound standards were purchased from Sigma-Aldrich and Avanti Polar Lipids (Alabaster, AL).
Experimental samples were prepared for GC–MS analysis as previously described [43]. Human serum samples were thawed at 4 °C. Next, 200 μL 10 × diluted PBS and 80 μL of MeOH containing 50 μM PC (17:0, 17:0) and PG (17:0, 17:0) internal standards were added to each thawed sample (20 μL for human serum). Afterward, 400 μL of MTBE was added to each sample (MTBE: MeOH: H2O = 10:2:5, v/v/v), vortexed for 30 s, and then stored at – 20 °C for 20 min. Lastly, samples were centrifuged at 21,300 g to separate aqueous and MTBE phases. The aqueous bottom layer (180 μL) from the MTBE extraction described above was collected into new Eppendorf tubes for derivatization prior to targeted metabolic profiling with GC–MS. The aqueous layer was dried under vacuum at 37 ºC for 4 h using a Savant SpeedVac vacuum concentrator. The residues were first derivatized with 40 µL of 20 mg/mL MeOX solution in pyridine under 60ºC for 90 min. Next, 60 µL of MTBSTFA containing d27-mysristic acid were added, and the mixture was incubated at 60 ºC for 30 min. The samples were then vortexed for 30 s, followed by centrifugation at 21,300 g for 10 min. Finally, 70 µL of supernatant were collected from each sample into new glass vials for GC–MS analysis while 10 µL of each sample was pooled to create a quality control (QC) sample, which was injected once every 10 experimental samples to monitor gradual changes in systems performance.
GC–MS conditions used here were mainly adopted from previous studies [30, 44]. Briefly, GC–MS experiments were performed on an Agilent 7820A GC-5977B MSD system (Santa Clara, CA) by injecting 1 µL of prepared samples. Research-grade helium (99.9995% purity) was used as the carrier gas with a constant flow rate of 1.2 mL/min. Front inlet, auxiliary line, and source temperatures were set to 250 ºC, 290 ºC, and 230 ºC, respectively. The separation of metabolites was achieved using an Agilent HP-5 ms capillary column (30 m × 250 µm × 0.25 µm). The column temperature was maintained at 60 °C for 1 min, increased at a rate of 10 °C/min to 325 °C, and then held at this temperature for 10 min. Data were recorded following a 3 min solvent delay. Electron energy was set to –70 eV, and mass spectral data were collected between m/z 60–550. Data extraction was performed using Agilent MassHunter Profinder software. Collected data were unbiasedly queried against an internal spectral and retention time library for the identification of the 126 targeted analytes and an RT tolerance of 0.10 min was used.
Statistical analysis
Data was analyzed using GraphPad Prism version 9.5.1 (GraphPad Software). Group differences for human serum samples were analyzed using a one-way analysis of variance (ANOVA) followed by Bonferroni’s corrected post hoc test, when appropriate. When human data points were missing, the subject was not included in the analysis. A non-parametric Kruskal–Wallis test was utilized when violations of homogeneity of variance were detected. Unpaired t-tests were used to analyze differences between the human AD groups and between the diet groups, when appropriate. Group differences for mouse samples were analyzed using two-way factorial ANOVAs (for genotype by diet). Linear correlations were calculated using the Pearson’s r coefficient and if subject data was missing for one of the two variables being analyzed, they were not included in the analysis. For metabolomics analysis, following peak integration, metabolites were filtered for reliability and only those with QC coefficient of variation (CV) < 20% and relative abundance of 1000 in > 80% of samples were retained for statistical analysis. Univariate and multivariate analyses of metabolomics data were performed using the MetaboAnalystR package [44]. A total of 184 missing values (4.7%) were detected in the human serum dataset. Metabolites with > 50% missing values were removed from analysis, and remaining missing values were imputed using a sample-wise k-nearest neighbors’ algorithm. Human serum samples were sum normalized, log10-transformed, and mean centered before analysis. Statistical outliers were identified and excluded using GraphPad Prism’s ROUT test. Significance was set at P < 0.05.
Results
Pathological AD hallmarks in human MCI subjects
We first assessed human MCI subjects on profile metrics. Analysis between the MCI Sparse and MCI High showed no significant differences in years since diagnosis (Supplemental Fig. 1a; t13 = 1.773, P = 0.0997) or age at diagnosis (Supplemental Fig. 1b; t9 = 0.5964, P = 0.5656). There were no significant differences between the groups (CON, MCI Sparse, MCI High; Supplemental Fig. 1c–f) for final BMI (F2,28 = 2.465, P = 0.1033), and time since last BMI (F2,28 = 2.460, P = 0.1037). There was a significant difference between the groups in expired age (F2, 29 = 4.947, P = 0.0142), where CON had a significantly lower expired age than MCI High cases (P = 0.0151). There were no significant group differences for PMI tissue collection (F2,29 = 2.722, P = 0.0825).
In MCI cases, serum choline and TNFα levels correlate with AD-associated pathologies. a–c Serum levels of choline, ACh, and TNFα. d Correlation between choline and ACh. e–g Correlations between MMSE and serum measurements. h–j Correlations between Global cognitive score and serum measurements. k–m Correlations between CERAD neuritic plaque density and serum measurements. 0 = Zero, S = Sparse, M = Moderate, F = Frequent. n–p Correlations between Braak stage and serum measurements. q–r Correlation between choline, ACh and TNFα. s–u Correlations between brain weight and serum measurements. v–x Choline, ACh and TNFα levels do not significantly differ between cases with and without cerebral amyloid angiopathy (CAA). y-aa Choline, ACh and TNFα levels did not significantly differ between cases with and without cerebral white matter rarefaction (CWMR). Data are reported as means ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001
We next analyzed the three groups (CON, MCI Sparse, MCI High) to assess differences in pathological burden. There was no significant group difference in cognition, as measured by the Mini Mental State Exam (MMSE; Supplemental Fig. 1g; F2,26 = 0.9884, P = 0.3857). We also assessed cognition with the Global cognitive score in MCI patients, which is based on a interview with an informant, with higher scores indicating worse impairments. There were no significant differences between the two MCI groups in Global cognitive score (MCI Sparse average score = 0.4286, MCI High average score = 0.546; Supplemental Fig. 1h; t16 = 0.6992, P = 0.4945). For CERAD neuritic plaque density, a semi-quantitative measure of deteriorating neuronal material surrounding Aβ plaques, we found significant group differences (Supplemental Fig. 1i; F2,29 = 72.27, P < 0.0001). MCI High had significantly higher plaque density than CON (P < 0.0001) and MCI Sparse (P < 0.0001). CON and MCI Sparse did not significantly differ in plaque burden (P > 0.9999). For Braak score, a measure of the extent of NFT pathology, we found significant group differences (Supplemental Fig. 1j; F2,29 = 31.50, P < 0.0001). MCI High had significantly higher Braak stage than CON (P < 0.0001) and MCI Sparse (P < 0.0001). CON and MCI Sparse did not significantly differ in Braak burden (P = 0.7460). Lastly, we analyzed brain weight and found no significant group differences (Supplemental Fig. 1k; F2,29 = 0.7282, P = 0.4914). Collectively, these results highlight the pathological differences associated with different stages of MCI.
Serum choline, acetylcholine (ACh), and TNFα levels differ between control and MCI groups and correlates with neuropathology
We next assessed serum levels of choline, ACh, and TNFα. We found significant group differences in serum choline levels (Fig. 1a; F2,29 = 6.689, P = 0.0041); both MCI Sparse (P < 0.0487) and MCI High (P = 0.0038) had lower choline levels than the CON group. MCI Sparse and MCI High did not significantly differ in choline levels (P > 0.9999). For serum ACh, we found a non-significant trend between the groups (Fig. 1b; F2,29 = 3.127, P = 0.0589); with a trend towards higher ACh in CON compared to MCI High (P = 0.0555). For TNFα, we found significant group differences (Fig. 1c; F2,29 = 1238, P < 0.0001); MCI Sparse (P < 0.0001) and MCI High (P < 0.0001) had higher levels than CON. Additionally, MCI High had higher serum TNFα than MCI Sparse (P < 0.0001). These results highlight a disease-associated reduction of choline and an increase in TNFα levels that correspond with MCI pathological severity.
Next, we performed correlations between serum choline, ACh, TNFα, MMSE score, Global cognitive score, CERAD neuritic plaque density, Braak stage, and brain weight. We found a significant positive correlation between choline and ACh (Fig. 1d; r30 = 0.8265, P < 0.0001). Choline, ACh, and TNFα were not significantly correlated with MMSE (Fig. 1e–g) or global cognitive score (Fig. 1h–j). We next performed correlations between CERAD neuritic plaque density and choline, ACh, and TNFα. We found a significant negative correlation with choline (Fig. 1k; r30 = − 0.3548, P = 0.0463); lower choline was associated with higher plaque density. There was no significant correlation between ACh and plaque density (Fig. 1l). There was a positive correlation that showed that as TNFα increased, so did plaque density (Fig. 1m; r30 = 0.7509, P < 0.0001).
Next, we performed correlations between serum choline, ACh, TNFα, and Braak stage. As with plaque density, we found highly significant negative correlations between choline (Fig. 1n; r30 = − 0.4020, P = 0.0226) and Braak stage, but not between ACh and Braak stage (Fig. 1o). A significant positive correlation illustrated that as TNFα increased, so did Braak stage (Fig. 1p; r30 = 0.6984, P < 0.0001). Further, both choline (Fig. 1q; r30 = − 0.5250, P = 0.0020) and ACh (Fig. 1r; r30 = − 0.3775, P = 0.0332) were significantly negatively correlated with TNFα. Lastly, there were no significant correlations between choline, ACh, TNFα and brain weight (Fig. 2s–u). These results highlight that in MCI, lower circulating choline, but not ACh, levels correlate with sub-diagnostic AD pathology, and that elevated TNFα is associated with increased Aβ and tau pathology.
Serum free choline, ACh and TNFα levels correlate with AD-associated pathologies in AD patients. a–c Serum levels of free choline, ACh, and TNFα. d Correlation between choline and ACh. e–g Correlations with MMSE and serum measurements. h–j Correlations with Global cognitive score and serum measurements. k–m Correlations with CERAD neuritic plaque density and serum measurements. 0 = Zero, S = Sparse, M = Moderate, F = Frequent. n–p Correlations between Braak stage and serum measurements. q–r Correlation between choline, ACh and TNFα. s–u Correlations between brain weight and serum measurements. Choline, ACh, and TNFα levels differ in cases with cerebral amyloid angiopathy (CAA) and cerebral white matter rarefaction (CWMR). v–x Choline and ACh are significantly reduced in CAA positive cases, while TNFα is increased. y-aa Choline is significantly reduced in cases with CWMR, but ACh and TNFα are not significantly different between CWMR cases. Data are reported as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
In patients with MCI, choline, ACh, and TNFα do not differ in cases with cerebral amyloid angiopathy (CAA) and cerebral white matter rarefaction (CWMR)
CAA is a condition where amyloid builds up in the walls of arteries and may lead to stroke and dementia [28]. To determine if choline, ACh and TNFα are significantly different in patients with confirmed CAA, we stratified human cases into those with and without CAA. We found that choline, ACh and TNFα were not significantly different in patients with CAA (Fig. 1v–x). Given that choline is important in the biosynthesis of phosphatidylcholine, a major metabolite of myelin, we next stratified human cases into those with or without CWMR, which is a measure of overall white matter integrity and myelin loss [1]. We then analyzed choline, ACh and TNFα serum between the CWMR categorized groups. We found that patients with CWMR did not significantly differ in choline, ACh, or TNFα levels (Fig. 1y-aa).
Pathological hallmarks in human AD subjects
We next analyzed human subject profile metrics of an independent cohort of cases including patients with AD compared to aged-matched controls. Analysis between the AD Mod and AD Sev showed no significant differences for years since diagnosis (Supplemental Fig. 2a; t22 = 1.046, P = 0.3068) or age at diagnosis (Supplemental Fig. 2b; t22 = 1.300, P = 0.2069). There were no significant differences between the groups (CON, AD Mod, AD Sev; Supplemental Fig. 2c–f) for final BMI (F2,28 = 0.5525, P = 0.5817), time since last BMI (F2,28 = 0.5406, P = 0.5884), expired age (F2,33 = 0.2349, P = 0.7920), and PMI tissue collection (F2, 33 = 0.8296, P = 0.4451).
We next analyzed the three groups (CON, AD Mod, AD Sev) to confirm differences in AD progression. There were significant group differences in cognition, as first measured by the Mini Mental State Exam (MMSE; Supplemental Fig. 2g; F2,26 = 15.68, P < 0.0001). AD Sev exhibited lower scores than CON (P < 0.0001) and AD Mod (P = 0.0059). We supported this with the Global cognitive score. A Kruskal–Wallis test identified significant group differences (Supplemental Fig. 2h; H2 = 7.178, P = 0.0194); AD Sev scores were significantly higher than CON (P = 0.0280). For CERAD neuritic plaque density, we found significant group differences (Supplemental Fig. 2i; F2,33 = 113.3, P < 0.0001). CON had significantly lower plaque density than AD Mod (P < 0.0001) and AD Sev (P < 0.0001). AD Mod trended towards lower plaque density than AD Sev (P = 0.0625). For Braak score, we found significant group differences (Supplemental Fig. 2j; F2,33 = 200.2, P < 0.0001). AD Sev had higher Braak stage classification than both CON (P < 0.0001) and AD Mod (P < 0.0001); AD Mod had higher Braak stage classification than CON (P < 0.0001). Lastly, we analyzed brain weight and found significant group differences (Supplemental Fig. 2k; F2,28 = 7.825, P = 0.0016). AD Sev brains weighed significantly less than CON (P = 0.0012). Collectively, these results highlight disease-associated changes between the AD Mod and AD Sev compared to CON subjects.
Serum choline, ACh, and TNFα levels differ between control and AD groups and correlates with neuropathology
We assessed serum levels of choline, ACh, and TNFα. We found significant group differences of serum choline levels (Fig. 2a; F2,33 = 62.45, P < 0.0001); both AD Mod (P = 0.0155) and AD Sev (P < 0.0001) had lower choline levels than CON. AD Sev had lower choline than AD Mod (P < 0.0001). For serum ACh, we found significant group differences (Fig. 2b; F2,33 = 204.2, P < 0.0001); both AD Mod (P < 0.0001) and AD Sev (P < 0.0001) had lower levels than CON. Additionally, AD Sev had lower ACh than AD Mod (P < 0.0001). For TNFα, we found significant group differences (Fig. 2c; F2,33 = 18.82, P < 0.0001); AD Mod (P = 0.0231) and AD Sev (P < 0.0001) had higher levels than CON. Additionally, AD Sev had higher serum TNFα than AD Mod (P = 0.0071). These results highlight a disease-associated reduction of choline and ACh, and an increase in TNFα levels in AD patients.
Next, we performed correlations between serum choline, ACh, TNFα, MMSE scores, Global cognitive score, CERAD neuritic plaque density, Braak stage, and brain weight. We found a significant positive correlation between choline and ACh (Fig. 2d; r30 = 0.7713, P < 0.0001). On measures of cognitive ability, we found significant correlations between both choline (Fig. 2e; r27 = 0.5936, P = 0.0007), ACh (Fig. 2f; r27 = 0.7064, P < 0.0001) and MMSE, indicating that higher choline and ACh are associated with higher MMSE outcomes. Conversely, TNFα was negatively correlated with MMSE (Fig. 2g; r27 = − 0.5275, P = 0.0033). As another metric of cognitive ability, we performed correlations with Global cognitive score (Fig. 2h-j). While choline levels were not significantly correlated with Global cognitive score, we found a significant positive correlation between ACh and Global cognitive score (r15 = -0.6313, P = 0.0066). Global cognitive score was not significantly correlated with TNFα. We next performed correlations between CERAD neuritic plaque density and choline, ACh, and TNFα. We found significant negative correlations between both choline (Fig. 2k; r34 = − 0.6674, P < 0.0001), ACh (Fig. 2l; r34 = − 0.8884, P < 0.0001) and plaque density; lower choline and ACh was associated with higher plaque density. Further, a positive correlation showed that as TNFα increased, so did plaque density (Fig. 2m; r34 = 0.5858, P = 0.0002).
Next, we performed correlations between serum choline, ACh, TNFα, and Braak stage. We found highly significant negative correlations between choline (Fig. 2n; r34 = -0.8394, P < 0.0001), ACh (Fig. 2o; r34 = − 0.9274, P < 0.0001) and Braak stage. A significant positive correlation illustrated that as TNFα increased, so did Braak stage (Fig. 2p; r34 = 0.7022, P < 0.0001). Further, there were significant negative correlations between both choline (Fig. 2q; r34 = − 0.6485, P < 0.0001), ACh (Fig. 2r; r34 = − 0.6853, P < 0.0001) and TNFα. Lastly, there were significant positive correlations for both choline (Fig. 2s; r34 = 0.5150, P = 0.0013), ACh (Fig. 2t; r34 = 0.5120, P = 0.0014) and brain weight, and a negative correlation between TNFα and brain weight (Fig. 2u; r34 = − 0.4684, P = 0.0040). These results highlight that higher choline and ACh levels correlate with lower AD pathology, and elevated TNFα is associated with increased AD pathology.
Choline, ACh, and TNFα differ in cases with cerebral amyloid angiopathy (CAA) and cerebral white matter rarefaction (CWMR)
To determine if choline, ACh and TNFα are significantly different in patients with confirmed CAA, we stratified human cases into those with and without CAA. We found that both choline (t34 = 3.830, P = 0.0005) and ACh (t34 = 4.257, P = 0.0002) are significantly lower in patients with CAA (Fig. 2v, w). We also found a significant increase in TNFα in patients with CAA (Fig. 2x; t34 = 3.335, P = 0.0021). We then analyzed choline, ACh and TNFα serum between the CWMR categorized groups. Interestingly, we found that patients with CWMR had lower choline (Fig. 2y; t34 = 2.055, P = 0.0476), however, there were no significant differences in ACh or TNFα between patients with or without CWMR (Fig. 2z, aa), suggesting that low circulating choline may be associated with a reduction in myelin, but not ACh nor TNFα.
A choline-deficient diet reduced choline and ACh levels in both 3xTg-AD and NonTg mice, with 3xTg-AD mice showing greater deficits
To determine the consequences of dietary choline deficiency, we placed 3xTg-AD and NonTg mice on either a choline normal (ChN) or choline deficient (Ch−) diet from three to 12 months of age (Fig. 3a). Notably, we previously published findings showing that food consumption in these mice did not vary between the diet groups (see [11]). Blood plasma was obtained from live mice at 7 and 12 months of age to measure choline, ACh, and TNFα. We found a significant main effect of age; plasma choline was decreased with age (Fig. 3b; F1,20 = 158.3, P < 0.0001). At 7 months of age, there was a main effect of genotype (F1,20 = 198.1, P < 0.0001), with lower choline in the 3xTg-AD mice compared to the NonTg mice. Additionally, there was a significant main effect of diet (F1,20 = 40.75, P < 0.0001), with lower choline in the Ch− mice compared to the ChN counterparts. At 12 months, we found a significant main effect of diet, where 3xTg-AD Ch− and NonTg Ch− mice showed reduced choline in both plasma (F1,20 = 96.08, P < 0.0001) and cortex (Fig. 3c; F1,20 = 165.3, P < 0.0001). The 12 month choline findings were previously reported [11] and are reiterated for clarity. For plasma ACh, we also found a significant main effect of age (Fig. 3d; F1,20 = 1335, P < 0.0001), where levels were decreased with age. At 7 months, we found significant main effects of both genotype (F1,20 = 348.2, P < 0.0001) and diet (F1,20 = 124.5, P < 0.0001), with lower ACh in 3xTg-AD mice and Ch− mice. Additionally, we found a significant genotype by diet interaction (F1,20 = 92.79, P < 0.0001); there was significantly less ACh in the 3xTg-AD Ch− mice compared to their ChN counterparts (P < 0.0001). There were no significant dietary differences in the NonTg mice. At 12 months, we found significant main effects of both genotype (F1,20 = 57.34, P < 0.0001) and diet (F1,20 = 22.77, P = 0.0001), where 3xTg-AD mice had lower ACh than NonTg and the Ch− diet led to reduced levels. We also found a significant genotype by diet interaction (F1,20 = 18.40, P = 0.0004), where NonTg Ch− mice had lower ACh than their ChN counterparts (P < 0.0001). Interestingly, both the 3xTg-AD ChN (P < 0.0001) and 3xTg-AD Ch− (P < 0.0001) mice had lower ACh than the NonTg ChN, indicating diet-independent reductions in 3xTg-AD mice. Taken together, a Ch− diet reduces plasma and cortical choline in both NonTg and 3xTg-AD mice. Interestingly, while only 3xTg-AD mice show substantial reduction of ACh following a Ch− at 7 months, by 12 months both 3xTg-AD mice groups and the NonTg Ch− mice show significantly reduced ACh.
A choline deficient (Ch−) diet results in reduced choline and ACh, but elevated TNFα at 7 and 12 months (mo). a Timeline of dietary choline manipulation. b At 7 mo, 3xTg-AD mice exhibit lower plasma choline than NonTg mice. Ch− led to decreases in plasma choline at both 7 and 12 mo. c At 12 mo, Ch− mice exhibited lower cortical choline levels. d At 7 mo, 3xTg-AD mice showed lower plasma ACh than NonTg mice and the 3xTg-AD Ch− mice had the lowest plasma ACh. At 12 mo, the Ch− diet led to a decrease in ACh in NonTg mice, and 3xTg-AD mice had lower ACh than NonTg regardless of diet. e At 7 and 12 mo, plasma TNFα was higher in the 3xTg-AD mice than in the NonTg mice and the Ch− diet increased TNFα even further. f 3xTg-AD mice had higher cortical TNFα than NonTg mice, and the Ch− diet increased levels in both genotypes. g–n Correlations between plasma choline, cortex choline, ACh, and TNFα in NonTg and 3xTg-AD mice at 12 mo. Data are reported as means ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001
We next examined TNFα in plasma and cortex. We found a significant main effect of age, indicating that plasma TNFα increases with age (Fig. 3e; F1,20 = 5.614, P = 0.0280). At 7 months, there were significant main effects of both genotype (F1,20 = 281.0, P < 0.0001) and diet (F1,20 = 9.462, P = 0.0060); 3xTg-AD mice had higher levels than NonTg mice and the Ch− diet groups had higher levels than ChN mice. There was also a significant genotype by diet interaction (F1,20 = 10.80, P = 0.0037), where the 3xTg-AD Ch− mice (P = 0.0013) had higher plasma TNFα than their ChN counterparts, but there was no significant diet difference between the NonTg mice. In plasma at 12 months of age, we found significant main effects of both genotype (F1,20 = 4754, P < 0.0001) and diet (F1,20 = 259.7, P < 0.0001), where the 3xTg-AD mice and the Ch− mice showed elevated TNFα. We also found a significant genotype by diet interaction (F1,20 = 168.3, P < 0.0001), where 3xTg-AD ChN mice had higher TNFα than both NonTg ChN (P < 0.0001) and NonTg Ch− (P < 0.0001) mice. Further, 3xTg-AD Ch− mice had significantly elevated TNFα compared to their ChN counterparts (P < 0.0001). In the cortex (Fig. 3f), we also observed significant main effects of both genotype (F1,27 = 247.2, P < 0.0001) and diet (F1,27 = 64.67, P < 0.0001) for TNFα, with 3xTg-AD mice and the Ch− mice exhibiting elevations. Collectively, this data highlights that the 3xTg-AD mice have higher TNFα than NonTg mice at both 7 and 12 months of age, and that the Ch− diet increases levels of this pro-inflammatory cytokine.
We next performed correlation analyses of our plasma and cortical measures in 12-month-old mice. Given the substantial genotypical group differences in ACh and TNFα, our correlation analyses were separated by genotype. We found significant positive correlations between plasma and cortical choline in both the NonTg (Fig. 3g; r10 = 0.8911, P < 0.0001) and 3xTg-AD mice (Fig. 3h; r10 = 0.8673, P = 0.0003). In the NonTg mice, there was a significant positive correlation between plasma choline and ACh (Fig. 3i; r10 = 0.7854, P = 0.0025), but was non-significant in 3xTg-AD mice (Fig. 3j). In both NonTg (Fig. 3k; r10 = − 0.9431, P < 0.0001) and 3xTg-AD (Fig. 3l; r10 = − 0.8854, P < 0.0001) mice, there were significant negative correlations between plasma choline and TNFα. There was also a significant negative correlation between ACh (Fig. 3m; r10 = − 0.8803, P = 0.0002) and TNFα for NonTg mice, but a non-significant correlation in 3xTg-AD mice (Fig. 3n). These results highlight the association between choline, its derivate ACh, and TNFα, and how, in 3xTg-AD mice, a Ch− diet dysregulates their circulating and cortical levels.
A choline-deficient diet exacerbates amyloidosis and tau phosphorylation in 3xTg-AD mice
Next, we examined the impact of a Ch− diet on pathological markers in the cortex of 3xTg-AD mice. For amyloidosis, we assessed soluble and insoluble Aβ (40 and 42), isoforms that aggregate to form Aβ plaques [11, 15]. For tau hyperphosphorylation, we analyzed phosphorylated tau epitopes that are pathological in AD, threonine (Thr) 181 and serine (S) 396 [11, 34, 45]. NonTg mice were excluded from this analysis because they do not harbor familial mutations leading to AD pathologies. Consistent with our previous report, we find that a Ch− diet did not increase soluble Aβ40, (Fig. 4a, left; t10 = 0.6704, P = 0.5178) [11]. We found significant elevations of soluble Aβ42 (Fig. 4a, right; t10 = 3.134, P = 0.0106), insoluble Aβ40 (Fig. 4b, left; t10 = 16.46, P < 0.0001) and Aβ42 (Fig. 4b, right; t10 = 16.83, P < 0.0001) in 3xTg-AD Ch− mice. We also found significantly higher soluble pTau Thr 181 (Fig. 4c, left; t10 = 19.12, P < 0.0001), insoluble pTau Thr 181 (Fig. 4c, right; t10 = 18.76, P < 0.0001), soluble pTau S 396 (Fig. 4d, left; t10 = 13.58, P < 0.0001), and insoluble pTau S 396 (Fig. 4d, right; t10 = 7.053, P < 0.0001) in 3xTg-AD Ch− mice. Collectively, these results highlight that a Ch− diet exacerbates amyloidosis and tau hyperphosphorylation, consistent with our previous report [11].
A choline deficient diet (Ch-) results in both elevated amyloidosis and hyperphosphorylated tau in 3xTg-AD mice. Correlations between choline, ACh, TNFα and the various forms of amyloid and pathological tau epitopes in 3xTg-AD mice. a–d In the cortex of 3xTg-AD mice, the Ch− diet elevated soluble Aβ42, insoluble Aβ40, insoluble Aβ42, soluble pTau Thr 181, insoluble pTau Thr 181, soluble pTau S 396, and insoluble pTau S 396. e–r Correlations between plasma choline, cortex choline, and soluble and insoluble Aβ40 and Aβ42, soluble and insoluble pTau Thr 181, and soluble and insoluble pTau S 396. s–y Correlations between plasma TNFα and soluble and insoluble Aβ40 and Aβ42, soluble and insoluble pTau Thr 181, and soluble and insoluble pTau S 396. Data are reported as means ± SEM. *P < 0.05, ****P < 0.0001
Next, we performed correlations between the AD-related amyloidosis and tau hyperphosphorylation with choline in 3xTg-AD mice. For soluble Aβ40, there were no significant correlations with cortical or plasma choline (Fig. 4e, f). For soluble Aβ42, there was no significant correlation with plasma choline (Fig. 4g), but a significant negative correlation with cortical choline (Fig. 4h; r10 = − 0.6561, P = 0.0205). For insoluble Aβ40, there were significant negative correlations for both plasma (Fig. 4i; r10 = − 0.8398, P = 0.0006) and cortical choline (Fig. 4j; r10 = − 0.9825, P < 0.0001). For insoluble Aβ42, there were significant negative correlations for both plasma (Fig. 4k; r10 = − 0.8329, P = 0.0008) and cortical choline (Fig. 4l; r10 = − 0.9706, P < 0.0001). For soluble pTau Thr 181, there were significant negative correlations for both plasma (Fig. 4m; r10 = − 0.8766, P = 0.0002) and cortical choline (Fig. 4n; r10 = − 0.9810, P < 0.0001). For insoluble pTau Thr 181, there were significant negative correlations for both plasma (Fig. 4o; r10 = − 0.8158, P = 0.0012) and cortical choline (Fig. 4p; r10 = − 0.9764, P < 0.0001). Additionally, for soluble pTau S 396, there were significant negative correlations for both plasma (Fig. 4q; r10 = − 0.8813, P = 0.0002) and cortical choline (Fig. 4r; r10 = − 0.9708, P < 0.0001). Lastly, for insoluble pTau S 396, there were significant negative correlations for both plasma (Fig. 4s; r10 = − 0.7858, P = 0.0024) and cortical choline (Fig. 4t; r10 = − 0.9217, P < 0.0001). There were no significant correlations between plasma ACh and Aβ or tau hyperphosphorylation within the various fractions.
Next, we looked at correlations of plasma TNFα with Aβ fractions and tau hyperphosphorylation. There was no significant correlation between soluble Aβ40 and TNFα (Fig. 4u), however, there were significant positive correlations between TNFα and soluble Aβ42 (Fig. 4v; r10 = 0.6544, P = 0.0210), insoluble Aβ40 (Fig. 4w; r10 = 0.9757, P < 0.0001) and insoluble Aβ42 (Fig. 4x; r10 = 0.9368, P < 0.0001). Additionally, there were significant positive correlations between TNFα and both soluble (Fig. 4y; r10 = 0.9813, P < 0.0001) and insoluble (Fig. 4z; r10 = 0.9517, P < 0.0001) pTau Thr 181. Lastly, there was a significant positive correlation between TNFα and both soluble (Fig. 4aa; r10 = 0.9691, P < 0.0001) and insoluble pTau S 396 (Fig. 4ab; r10 = 0.8938, P < 0.0001). These results highlight that a Ch− diet in the 3xTg-AD mice increased amyloidosis and tau hyperphosphorylation that are consistent with changes observed in human AD specimens, reflecting the impact of choline deficiency in disease progression.
Choline supplementation in the APP/PS1 mouse increases choline and ACh in the brain while reducing TNFα and amyloidosis
To determine the effects of dietary choline supplementation throughout life, APP/PS1 and NonTg mice were placed on a choline supplemented (Ch+) or standard control (Ctl) diet from 2.5 to 12.5 months of age. We then measured brain levels of choline, ACh and TNFα (Fig. 5a). We previously published findings which showed that these mice did not differ in body weight between the diet groups (see [58]). We found significant main effects of genotype (Fig. 5b; F1,19 = 39.95, P < 0.0001) and diet (F1,19 = 78.85, P < 0.0001) on choline levels, where APP/PS1 mice showed reductions compared to NonTg mice, and Ch+ mice showed elevations compared to their Ctl counterparts. We also found a significant genotype by diet interaction (F1,19 = 14.45, P = 0.0012), where the APP/PS1 Ch+ mice showed higher brain choline than APP/PS1 Ctl (P = 0.0066) and NonTg Ch+ show higher levels than NonTg Ctl (P < 0.0001). Notably, the brain choline levels in NonTg Ctl and APP/PS1 Ch+ (P = 0.488) did not significantly differ, illustrating a rescue effect. For brain ACh, we found significant main effects of genotype (Fig. 5c; F1,19 = 42.37, P < 0.001) and diet (F1,19 = 108.6, P < 0.0001), where APP/PS1 mice showed reductions compared to NonTg mice, and Ch+ mice showed elevations compared to their Ctl counterparts. We found significant main effects of genotype (Fig. 5d; F1,19 = 270.9, P < 0.0001) and diet (F1,19 = 76.75, P < 0.0001) for brain TNFα, where APP/PS1 mice showed elevations compared to NonTg mice, and Ch+ mice showed reductions compared to their Ctl counterparts. We also found a significant genotype by diet interaction (F1,19 = 120.9, P < 0.0001), where the APP/PS1 Ch+ mice showed significantly lower TNFα than APP/PS1 Ctl (P < 0.0001). These results illustrate that supplementing with choline can produce protective effects in the brain.
Choline supplementation in the APP/PS1 mouse increases choline and acetylcholine (ACh) in the brain while reducing TNFα and amyloidosis. a Timeline of dietary choline manipulation. b, c APP/PS1 mice show reduced levels of choline and ACh, and the Ch+ diet increased the levels in both NonTg and APP/PS1 mice. d APP/PS1 mice show increased levels of brain TNFα, and the Ch+ diet decrease the levels. e–g Correlations between choline, ACh and TNFα. h–s Correlations between brain choline, ACh, TNFα and soluble and insoluble Aβ40 and Aβ42. Data are reported as means ± SEM. **P < 0.01, ****P < 0.0001
We next performed correlation analyses between our brain measures in APP/PS1 and NonTg mice. We found a significant positive correlation between brain choline and ACh (Fig. 5e; r21 = 0.9189, P < 0.0001). We also found significant negative correlations between both choline (Fig. 5f; r21 = − 0.5579, P = 0.0057) and ACh (Fig. 5g; r21 = − 0.6606, P = 0.0006) with TNFα. Next, we performed correlations of soluble and insoluble Aβ40 and Aβ42 in the APP/PS1 mice. There were significant negative correlations between soluble Aβ40 and both choline (Fig. 5h; r11 = − 0.6346, P = 0.0198) and ACh (Fig. 5i; r11 = − 0.7137, P = 0.0062). There was no significant correlation between soluble Aβ42 and choline (Fig. 5j), but a trend toward significance between soluble Aβ42 and ACh (Fig. 5k; r11 = − 0.5421, P = 0.0556). Additionally, there were no significant correlations between insoluble Aβ40 and both choline and ACh (Fig. 5l, m). For insoluble Aβ42, there were significant negative correlations with both choline (Fig. 5n; r11 = − 0.5532, P = 0.0499) and ACh (Fig. 5o; r11 = − 0.6037, P = 0.0289). Lastly, there were significant positive correlations between TNFα with soluble Aβ40 (Fig. 5p; r11 = 0.6764, P = 0.0111) and insoluble Aβ40 (Fig. 5q; r11 = 0.5886, P = 0.0343). There was a trending positive correlation between TNFα and soluble Aβ42 (Fig. 5r), but no correlation with insoluble Aβ42 (Fig. 5s). These results highlight the association between choline, ACh, and TNFα, and how a Ch + diet in APP/PS1 can reduce the levels of various forms of soluble and insoluble amyloid fractions.
Metabolomics analysis reveals four key metabolites significantly correlated with choline
To investigate the metabolic differences in human subjects with the varying severities of AD versus CON, we conducted a comprehensive targeted metabolomic profile of metabolites from human serum and probed for associations with choline (Supplemental Fig. 3). Correlation analysis identified four metabolites that were significantly correlated with choline levels (Fig. 6a). There was a significant negative correlation of choline with L-Valine (Fig. 6b; r33 = − 0.442, P = 0.0079). Three metabolites showed a significant positive correlation with choline, including 4-Hydroxyphenylpyruvic acid (4-HPPA; Fig. 6c; r34 = 0.4528, P = 0.0056), Methylmalonic acid (MMA; Fig. 6d; r34 = 0.3653, P = 0.0285), and Ferulic acid (Fig. 6e; r34 = 0.3333, P = 0.047). We next examined the correlation of ACh with the metabolites and found a significant negative correlation of ACh with L-Valine (Fig. 6f; r33 = − 0.488, P = 0.0029) and positive correlations of ACh with 4-HPPA (Fig. 6g; r34 = 0.4066, P = 0.0139). The correlation between ACh and MMA was non-significant (Fig. 6h). There was a significant positive correlation between ACh and Ferulic acid (Fig. 6i; r34 = 0.3806, P = 0.022). Collectively, these results provide novel insights into the metabolic alterations associated with both choline and ACh levels.
Metabolomic analysis from human serum reveals metabolites that correlate with choline and acetylcholine (ACh). a Heatmap illustrating the relative abundance of each of the four metabolites significantly correlated with choline per sample. b A significant negative correlation between circulating choline levels and L-Valine. c–e Significant positive correlations between circulating choline levels and 4-Hydroxyphenylpyruvic acid, Methylmalonic acid and Ferulic acid. f A significant negative correlation between circulating ACh levels and L-Valine. g–i Significant positive correlations between circulating ACh levels and 4-Hydroxyphenylpyruvic acid and Ferulic acid, and a non-significant trend with Methylmalonic acid
Discussion
We found disease-associated reductions in serum choline and ACh in AD patients compared to their healthy aged-matched controls. Conversely, serum TNFα levels were elevated in all AD cases compared to healthy controls and the highest levels were associated with highest pathological burden. Additionally, we assessed the relationship between choline and other metrics of brain health. CAA cases displayed lower serum choline and ACh, and higher TNFα. Further, CWMR cases had lower choline than cases without CWMR, illustrating the importance of choline for white matter integrity. Importantly, our MCI cases showed reduced circulating choline and elevated TNFα levels that were associated with pathology, but ACh levels were unchanged. This suggests that initial disease development of Aβ and tau may be linked to choline reductions, but it is only later in disease progression that other pathologies, such as reductions in brain weight, CAA, and CWRM, become associated to choline levels. It also highlights that circulating ACh changes accompany AD, but not MCI. Previous research that found relationships between decreased choline intake and increased risk for cognitive decline and AD development estimated intake levels and did not measure pathology [38, 68]. Consequently, this is the first study examining the relationship between circulating choline levels and pathological assessments of human brain tissue in both MCI and AD cases at various phases of pathological progression, highlighting the importance of monitoring choline for brain health.
We examined the impact of dietary choline deficiency and supplementation on pathogenesis in two mouse models of AD and found parallels to our human findings. Higher TNFα, Aβ, and tau phosphorylation were correlated with lower plasma choline levels due to the dietary deficiency in the 3xTg-AD mice. Notably, the Ch− diet resulted in lower plasma choline and ACh, and elevated TNFα at 7 months, an age when amyloidosis commences in 3xTg-AD mice, but prior to NFT pathology. Thus, dietary choline differences predate and contribute to pathology; at 7 months, there was less plasma choline in the 3xTg-AD mice than the NonTg mice. Conversely, dietary choline supplementation in APP/PS1 mice elevated choline and ACh while reducing both TNFα and soluble and insoluble Aβ levels in the brain. A caveat of the APP/PS1 results is that multiple brain regions were included in the analysis, and a specific brain region may drive these results. Together, this data provides evidence that higher levels of circulating choline correspond with reduced AD-related Aβ levels and tau hyperphosphorylation.
Elevated L-Valine, an essential amino acid, has been linked to a variety of disease-associated processes, including oxidative stress and insulin resistance [26]. While elevated L-Valine increases the risk of AD in some studies [53], other studies have shown lower L-Valine in AD [48]. Interestingly, a high choline diet reduces circulating L-Valine levels, likely through increased catabolism or uptake by muscles [2]. We found that higher choline and ACh are associated with lower L-Valine; the lowest L-Valine levels were in the CON and AD Mod groups, which supports previous evidence that reduced L-Valine is protective against AD [48], and further indicating that reducing L-Valine may be a protective action of choline against AD.
Two identified metabolites correlating with choline and ACh levels have anti-acetylcholinesterase activity, which prevents enzymatic breakdown of ACh. 4-HPPA, an amino acid metabolite, has high specificity and inhibitory activity for acetylcholinesterase [9, 54]. Ferulic acid is a plant-derived antioxidant that’s been shown to improve AD pathology [47, 66]. Ferulic acid also has anti-acetylcholinesterase activity and increases ACh [47, 66]. Interestingly, many of the first line drugs to treat AD are acetylcholinesterase inhibitors [54], suggesting these metabolites can be particularly beneficial against AD reductions in ACh. Notably, serum ACh was positively correlated with 4-HPPA and Ferulic acid, supporting the anti-acetylcholinesterase activity. Indeed, this data suggests elevated choline may have a dual protective role; it both increases the available choline for synthesizing ACh and increases metabolites with anti-acetylcholinesterase activity.
We found a significant positive correlation between MMA and choline levels, but not ACh levels. MMA is a precursor to succinyl-CoA, which is a key participant in the citric acid cycle and a major source of ATP and energy [25]. The citric acid cycle is dysregulated in AD [50]. We found that those with the lowest choline levels corresponded to lower MMA. Metabolomic studies have found that MMA is decreased in patients with MCI and AD compared to healthy controls [19]. This supports our findings as the AD Sev group had the lowest choline levels. Other evidence however suggests that elevated MMA may be a risk factor for disease states [39], including dementia [7]. While studies show that choline and MMA levels are inversely related [36, 39], a study on maternal choline supplementation in mice showed a small, but significant increase of MMA in choline supplemented dams [36], consistent with our findings. While our results are consistent with some results about MMA and its relationship with AD and choline, the literature is mixed, and future studies should further clarify the relationship between MMA and choline in AD.
Other recent studies support our findings that choline is decreased in the serum and brain of AD patients compared to healthy controls [13, 22, 24, 56]. However, others have observed increases in circulating choline, particularly in recently diagnosed AD patients [17, 21, 46], which may reflect neuronal membrane degradation, releasing choline from choline-containing phospholipids (CCPLs) [10, 21, 23, 27, 46]. Indeed, studies have shown that brain lipid composition changes are modified in AD, with specific reductions in CCPLs [23, 27, 49]. Reductions in free choline reduces the capacity to maintain both CCPLs and ACh [41, 65]. Cells prioritize ACh synthesis over CCPLs, leading to “autocannibalism” of CCPLs to free choline for ACh production [37, 65]. Only cholinergic neurons use their CCPLs as choline reserves for ACh production [41], and it is likely that reserves are diminished with disease progression leading to choline levels reflecting dietary intake and endogenous production. Here, we found that circulating choline is reduced in MCI and AD cases, but that ACh is only reduced in AD cases. This is supported by findings that choline acetyltransferase activity is most reduced in cases with severe AD [62]. This could reflect that compensatory measures are keeping ACh levels stable in MCI cases, but that the mechanisms might be depleted in the AD cases. Regardless, increased choline intake may prevent or delay the “autocannibalism” of CCPLs, preserving neuronal integrity.
Our human subject profiles did not include information about dietary habits, which has been documented in previous studies linking low choline with AD [38, 56, 68]. While we didn’t find significant differences in BMI between our human groups, appetite loss is common in AD [35]. Often caregivers of patients choose enjoyable foods over nutritional foods [35], suggesting that overall calories may be similar between groups, but nutritional values, including choline, might be lower in AD than healthy controls. Notably, an overall reduction of food consumption has been shown to result in reduced circulating choline within days [51]. Reduced circulating choline was clearly associated with greater pathology in the current study, which may be influenced by food consumption, PEMT variation and choline need throughout various organ systems. Future work in humans should establish how dietary choline intake and PEMT variation alters circulating choline levels, allowing for a complete assessment of its contributions to pathogenesis.
In conclusion, low circulating choline levels were associated with increased inflammation and neuropathology, suggesting that both consuming adequate dietary choline and monitoring circulating choline levels should be implemented for healthy aging. While adequate or even supplementation of dietary choline intake may not be able to reduce plaques or tangles when provided at an advanced stage of AD, it is critical for proper body and brain function throughout adulthood, serving as a preventive strategy to dampen AD pathological progression.
Data availability
Data are available in the main text or the supplementary materials. Raw data supporting the conclusions of this work will be made available upon reasonable request.
References
Alosco ML, Stein TD, Tripodis Y, Chua AS, Kowall NW, Huber BR et al (2019) Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol 76:1298–1308. https://doi.org/10.1001/JAMANEUROL.2019.2244
Alshaikh B, Schall JI, Maqbool A, Mascarenhas M, Bennett MJ, Stallings VA (2016) Choline supplementation alters some amino acid concentrations with no change in homocysteine in children with cystic fibrosis and pancreatic insufficiency. Nutr Res 36:418. https://doi.org/10.1016/J.NUTRES.2015.12.014
Alzheimer’s Association (2023) Alzheimer’s disease facts and figures. Alzheimers Dement 19:1598–1695
Arnold SJ, Dugger BN, Beach TG (2013) TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: Correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126:51–57. https://doi.org/10.1007/S00401-013-1110-0/TABLES/3
Ash JA, Velazquez R, Kelley CM, Powers BE, Ginsberg SD, Mufson EJ et al (2014) Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol Dis 70:32–42. https://doi.org/10.1016/J.NBD.2014.06.001
Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG et al (2015) Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35:354. https://doi.org/10.1111/NEUP.12189
Bednarska-Makaruk M, Graban A, Sobczyńska-Malefora A, Harrington DJ, Mitchell M, Voong K et al (2016) Homocysteine metabolism and the associations of global DNA methylation with selected gene polymorphisms and nutritional factors in patients with dementia. Exp Gerontol 81:83–91. https://doi.org/10.1016/J.EXGER.2016.05.002
Bi XH, Zhao HL, Zhang ZX, Zhang JW (2012) PEMT G523A (V175M) is associated with sporadic Alzheimer’s disease in a Chinese population. J Mol Neurosci 46:505–508. https://doi.org/10.1007/s12031-011-9630-3
Budryn G, Majak I, Grzelczyk J, Szwajgier D, Rodríguez-Martínez A, Pérez-Sánchez H (2022) Hydroxybenzoic acids as acetylcholinesterase inhibitors: calorimetric and docking simulation studies. Nutrients 14:2476. https://doi.org/10.3390/NU14122476
Cui Y, Liu X, Wang M, Liu L, Sun X, Ma L et al (2014) Lysophosphatidylcholine and amide as metabolites for detecting alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J Neuropathol Exp Neurol 17:954–963. https://doi.org/10.1097/NEN.0000000000000116
Dave N, Judd JM, Decker A, Winslow W, Sarette P, Villarreal Espinosa O et al (2023) Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer’s disease hallmarks. Aging Cell. https://doi.org/10.1111/acel.13775
Dave N, Vural AS, Piras IS, Winslow W, Surendra L, Winstone JK et al (2021) Identification of retinoblastoma binding protein 7 (Rbbp7) as a mediator against tau acetylation and subsequent neuronal loss in Alzheimer’s disease and related tauopathies. Acta Neuropathol 142:279–294. https://doi.org/10.1007/s00401-021-02323-1
de Wilde MC, Vellas B, Girault E, Yavuz AC, Sijben JW (2017) Lower brain and blood nutrient status in Alzheimer’s disease: results from meta-analyses. Alzheimer’s Dementia 3:416–431. https://doi.org/10.1016/J.TRCI.2017.06.002
Decourt B, Lahiri DK, Sabbagh MN (2017) targeting tumor necrosis factor alpha for Alzheimer’s disease HHS public access. Curr Alzheimer Res 14:412–425. https://doi.org/10.2174/1567205013666160930110551
Deture MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:1–18
Dorninger F, Moser AB, Kou J, Wiesinger C, Forss-Petter S, Gleiss A et al (2018) Alterations in the plasma levels of specific choline phospholipids in Alzheimer’s disease mimic accelerated aging. J Alzheimer’s Dis 62:841–854. https://doi.org/10.3233/JAD-171036
Elble R, Giacobini E, Higgins C (1989) Choline levels are increased in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging 10:45–50. https://doi.org/10.1016/S0197-4580(89)80009-0
Ferreira-Vieira HT, Guimaraes MI, Silva RF, Ribeiro MF (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115. https://doi.org/10.2174/1570159x13666150716165726
François M, Karpe AV, Liu JW, Beale DJ, Hor M, Hecker J et al (2022) Multi-omics, an integrated approach to identify novel blood biomarkers of Alzheimer’s disease. Metabolites. https://doi.org/10.3390/metabo12100949
Goh YQ, Cheam G, Wang Y (2021) Understanding choline bioavailability and utilization: first step toward personalizing choline nutrition. Cite This: J Agric Food Chem 69:10774–10789. https://doi.org/10.1021/acs.jafc.1c03077
González-Domínguez R, García A, García-Barrera T, Barbas C, Gómez-Ariza JL (2014) Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis–mass spectrometry. Electrophoresis 35:3321–3330. https://doi.org/10.1002/ELPS.201400196
González-Domínguez R, García-Barrera T, Luis Gómez-Ariza J (2014) Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer’s disease. doi: https://doi.org/10.1016/j.jprot.2014.01.014
Grimm MOW, Grösgen S, Riemenschneider M, Tanila H, Grimm HS, Hartmann T (2011) From brain to food: analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin–plasmalogens derivates in Alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J Chromatogr A 1218:7713–7722. https://doi.org/10.1016/J.CHROMA.2011.07.073
Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR et al (2011) Metabolomics in early Alzheimer’s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE 6:e21643. https://doi.org/10.1371/JOURNAL.PONE.0021643
Herrmann W, Schorr H, Bodis M, Knapp JP, Müller A, Stein G et al (2000) Role of homocysteine, cystathionine and methylmalonic acid measurement for diagnosis of vitamin deficiency in high-aged subjects. Eur J Clin Invest 30:1083–1089. https://doi.org/10.1046/J.1365-2362.2000.00746.X
Hu W, Yang P, Fu Z, Wang Y, Zhou Y, Ye Z et al (2022) High l-valine concentrations associate with increased oxidative stress and newly-diagnosed type 2 diabetes mellitus: a cross-sectional study. Diab Metab Synd Obesity 15:499–509. https://doi.org/10.2147/DMSO.S336736
Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS (2011) Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimer’s Dis 24:507–517. https://doi.org/10.3233/JAD-2011-101608
Jäkel L, De Kort AM, Klijn CJM, Schreuder FHBM, Verbeek MM (2022) Prevalence of cerebral amyloid angiopathy: a systematic review and meta-analysis. Alzheimer’s Dementia 18:10–28. https://doi.org/10.1002/ALZ.12366
Jankowsky JL, Zheng H (2017) Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol Neurodegener 12:1–22. https://doi.org/10.1186/s13024-017-0231-7
Jasbi P, Mohr AE, Shi X, Mahmood T, Zhu Q, Bruening M et al (2022) Whisner C (2022) Microbiome and metabolome profiles of high screen time in a cohort of healthy college students. Scient Rep 12:1–17. https://doi.org/10.1038/s41598-022-07381-3
Jasbi P, Shi X, Chu P, Elliott N, Hudson H, Jones D et al (2021) Metabolic profiling of neocortical tissue discriminates Alzheimer’s Disease from mild cognitive impairment, high pathology controls, and normal controls. Cite This: J Proteome Res 20:4303–4317. https://doi.org/10.1021/acs.jproteome.1c00290
Javonillo DI, Tran KM, Phan J, Hingco E, Kramár EA, da Cunha C et al (2022) Systematic phenotyping and characterization of the 3xTg-AD mouse model of Alzheimer’s disease. Front Neurosci 15:1–17. https://doi.org/10.3389/fnins.2021.785276
Jia JP, Jia JM, Zhou WD, Xu M, Chu CB, Yan X et al (2004) Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Chin Med J (Engl) 117:1161–1164
Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL et al (2020) Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19:422–433. https://doi.org/10.1016/S1474-4422(20)30071-5
Keller HH, Edward HG, Cook C (2007) Mealtime experiences of families with dementia. Am J Alzheimer’s Dis Other Dement 21:431–438. https://doi.org/10.1177/1533317506294601
King JH, Kwan ST, Bae S, Klatt KC, Yan J et al (2019) Maternal choline supplementation alters vitamin B-12 status in human and murine pregnancy. J Nutr Biochem 72:108210. https://doi.org/10.1016/J.JNUTBIO.2019.07.001
Klein J (2000) Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids Review Article. J Neural Transm 107:1027–1063
Liu L, Qiao S, Zhuang L, Xu S, Chen L, Lai Q et al (2021) Choline intake correlates with cognitive performance among elder adults in the United States. Behav Neurol. https://doi.org/10.1155/2021/2962245
Liu X, Gao X, Zhang R, Liu Z, Shen N, Di Y et al (2020) Discovery and comparison of serum biomarkers for diabetes mellitus and metabolic syndrome based on UPLC-Q-TOF/MS. Clin Biochem 82:40–50. https://doi.org/10.1016/J.CLINBIOCHEM.2020.03.007
Lockman PR, Allen DD (2002) The transport of choline. Drug Dev Ind Pharm 28:749–771. https://doi.org/10.1081/DDC-120005622
Maire JCE, Wurtman RJ (1984) Choline production from choline-containing phospholipids: a hypothetical role in Alzheimer’s disease and aging. Prog Neuropsychopharmacol Biol Psychiatry 8:637–642. https://doi.org/10.1016/0278-5846(84)90027-7
Mifflin MA, Winslow W, Surendra L, Tallino S, Vural A, Velazquez R (2021) Sex differences in the Intellicage and the morris water maze in the APP/PS1 mouse model of amyloidosis. Neurobiol Aging 101:130–140. https://doi.org/10.1016/J.NEUROBIOLAGING.2021.01.018
Mohr AE, Jasbi P, Bowes DA, Dirks B, Whisner CM, Arciero KM et al (2022) Exploratory analysis of one versus two-day intermittent fasting protocols on the gut microbiome and plasma metabolome in adults with overweight/obesity. Front Nutr 9:1–19. https://doi.org/10.3389/fnut.2022.1036080
Mohr AE, Jasbi P, Vander Wyst KB, van Woerden I, Shi X, Gu H et al (2022) Association of food insecurity on gut microbiome and metabolome profiles in a diverse college-based sample. Scient Rep 12:1–16. https://doi.org/10.1038/s41598-022-18515-y
Mondragón-Rodríguez S, Perry G, Luna-Muñoz J, Acevedo-Aquino MC, Williams S (2014) Phosphorylation of tau protein at sites Ser396-404 is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol Appl Neurobiol 40:121–135. https://doi.org/10.1111/nan.12084
Peña-Bautista C, Roca M, Hervás D, Cuevas A, López-Cuevas R, Vento M et al (2019) Plasma metabolomics in early Alzheimer’s disease patients diagnosed with amyloid biomarker. J Proteomics 200:144–152. https://doi.org/10.1016/J.JPROT.2019.04.008
Phadke AV, Tayade AA, Khambete MP (2021) Therapeutic potential of ferulic acid and its derivatives in Alzheimer’s disease—a systematic review. Chem Biol Drug Des 98:713–721. https://doi.org/10.1111/CBDD.13922
Polis B, Samson AO (2020) Role of the metabolism of branched-chain amino acids in the development of Alzheimer’s disease and other metabolic disorders. Neural Regen Res 15:1460–1470. https://doi.org/10.4103/1673-5374.274328
Rothhaar TL, Grösgen S, Haupenthal VJ, Burg VK, Hundsdörfer B, Mett J et al (2012) Plasmalogens inhibit APP processing by directly affecting γ-secretase activity in Alzheimer’s disease. Scient World J 2012:15. https://doi.org/10.1100/2012/141240
Sang C, Philbert SA, Hartland D, Unwin RD, Dowsey AW, Xu J et al (2022) Coenzyme A-dependent tricarboxylic acid cycle enzymes are decreased in Alzheimer’s disease consistent with cerebral pantothenate deficiency. Front Aging Neurosci 14:1–13. https://doi.org/10.3389/fnagi.2022.893159
Savendahl L, Mar MH, Underwood LE, Zeisel SH (1997) Prolonged fasting in humans results in diminished plasma choline concentrations but does not cause liver dysfunction. Am J Clin Nutr 66:622–625. https://doi.org/10.1093/AJCN/66.3.622
Scheltens P, de Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE et al (2021) Alzheimer’s disease. The Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Siddik MAB, Mullins CA, Kramer A, Shah H, Gannaban RB, Zabet-Moghaddam M et al (2022) Branched-chain amino acids are linked with Alzheimer’s disease-related pathology and cognitive deficits. Cells. https://doi.org/10.3390/cells11213523
Szwajgier D (2015) Anticholinesterase activity of selected phenolic acids and flavonoids—interaction testing in model solutions. Ann Agric Environ Med 22:690–694. https://doi.org/10.5604/12321966.1185777
Tohgi H, Abe T, Kimura M, Saheki M, Takahashi S (1996) Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J Neural Transm 103:1211–1220. https://doi.org/10.1007/BF01271206/METRICS
van Wijk N, Slot RER, Duits FH, Strik M, Biesheuvel E, Sijben JWC et al (2017) Nutrients required for phospholipid synthesis are lower in blood and cerebrospinal fluid in mild cognitive impairment and Alzheimer’s disease dementia. Alzheimer’s Dementia 8:139–146. https://doi.org/10.1016/j.dadm.2017.04.005
Velazquez R, Ash JA, Powers BE, Kelley CM, Strawderman M, Luscher ZI et al (2013) Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 58:92–101. https://doi.org/10.1016/J.NBD.2013.04.016
Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W et al (2019) Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 18:13037. https://doi.org/10.1111/acel.13037
Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S et al (2017) Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer’s disease. Neurobiol Aging 58:1–13. https://doi.org/10.1016/j.neurobiolaging.2017.06.003
Vennemann FBC, Ioannidou S, Valsta LM, Dumas C, Ocké MC, Mensink GBM et al (2015) Dietary intake and food sources of choline in European populations. Br J Nutr 114:2046–2055. https://doi.org/10.1017/S0007114515003700
Wang Y, Guan X, Chen X, Cai Y, Ma Y, Ma J et al (2019) Choline supplementation ameliorates behavioral deficits and Alzheimer’s disease-like pathology in transgenic APP/PS1 mice. Mol Nutr Food Res 63:1801407. https://doi.org/10.1002/MNFR.201801407
Wilcock GK, Esiri MM, Bowen DM, Smith CCT (1982) Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. Neurol Sci 57:407–117
Winslow W, McDonough I, Tallino S, Decker A, Vural AS, Velazquez R (2021) IntelliCage automated behavioral phenotyping reveals behavior deficits in the 3 × Tg-AD mouse model of alzheimer’s disease associated with brain weight. Front Aging Neurosci 13:506. https://doi.org/10.3389/FNAGI.2021.720214/BIBTEX
Winstone JK, Pathak KV, Winslow W, Piras IS, White J, Sharma R et al (2022) Glyphosate infiltrates the brain and increases pro-inflammatory cytokine TNFα: implications for neurodegenerative disorders. J Neuroinflammation 19:1–14. https://doi.org/10.1186/S12974-022-02544-5/FIGURES/5
Wurtman RJ, Blusztajn JK, Maire JC (1985) “Autocannibalism” of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease-a hypothesis. Neurochem Int 7:369–372. https://doi.org/10.1016/0197-0186(85)90127-5
Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO et al (2001) Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol 133:89–96. https://doi.org/10.1038/SJ.BJP.0704047
Ylilauri MPT, Voutilainen S, Lönnroos E, Virtanen HEK, Tuomainen TP, Salonen JT et al (2019) Associations of dietary choline intake with risk of incident dementia and with cognitive performance: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 110:1416–1423. https://doi.org/10.1093/AJCN/NQZ148
Yuan J, Liu X, Liu C, Ang AFA, Massaro J, Devine SA et al (2022) Is dietary choline intake related to dementia and Alzheimer’s disease risks? Results from the Framingham Heart Study. Am J Clin Nutr 116:1201–1207. https://doi.org/10.1093/ajcn/nqac193
Zeisel SH (2017) Choline, other methyl-donors and epigenetics. Nutrients 9:1–10. https://doi.org/10.3390/nu9050445
Acknowledgements
We would like to thank Savannah Tallino for assisting with figure design. We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human biological materials.
Funding
This work was supported by grants to Ramon Velazquez from the National Institute on Aging (R01 AG059627 and R01 AG062500). The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 and P30AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05–901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Author information
Authors and Affiliations
Contributions
Conceptualization: RV, JMJ. Investigation: JMJ, WW, PJ. Visualization: JMJ, RV, PJ. Supervision: RV, JKS. Writing—original draft: JMJ. Writing—review & editing: JMJ, PJ, WW, GES, TGB, JKS, RV.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Judd, J.M., Jasbi, P., Winslow, W. et al. Inflammation and the pathological progression of Alzheimer’s disease are associated with low circulating choline levels. Acta Neuropathol 146, 565–583 (2023). https://doi.org/10.1007/s00401-023-02616-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-023-02616-7