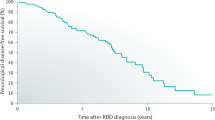
Abstract
Probable rapid eye movement (REM) sleep behavior disorder (pRBD) is a synucleinopathy-associated parasomnia in which loss of REM sleep muscle atonia results in motor behavior during REM sleep, including dream enactment. Traumatic brain injury is independently associated with increased risk of pRBD and Lewy body disease, and both pRBD and Lewy body disease are often observed in chronic traumatic encephalopathy (CTE). However, the frequency and pathological substrate of pRBD in CTE have not been formally studied and remain unknown. Of the total sample of 247 men, age at death of 63.1 ± 18.8 years (mean ± SD), 80 [32%] were determined by informant report to have symptoms of pRBD. These participants had played more years of contact sports (18.3 ± 11.4) than those without pRBD (15.1 ± 6.5; P = 0.02) and had an increased frequency of Lewy body disease (26/80 [33%] vs 28/167 [17%], P = 0.005). Of the 80 participants with pRBD, 54 [68%] did not have Lewy body disease; these participants were more likely to have neurofibrillary tangles and pretangles in the dorsal and median raphe (41 of 49 [84%] non-LBD participants with pRBD symptoms vs 90 of 136 [66%] non-LBD participants without pRBD symptoms, P = 0.02), brainstem nuclei with sleep regulatory function. Binary logistic regression modeling in the total study sample showed that pRBD in CTE was associated with dorsal and median raphe nuclei neurofibrillary tangles (OR = 3.96, 95% CI [1.43, 10.96], P = 0.008), Lewy body pathology (OR = 2.36, 95% CI [1.18, 4.72], P = 0.02), and years of contact sports participation (OR = 1.04, 95% CI [1.00, 1.08], P = 0.04). Overall, pRBD in CTE is associated with increased years of contact sports participation and may be attributable to Lewy body and brainstem tau pathologies.

Similar content being viewed by others
References
Adams JW, Alvarez VE, Mez J, Huber BR, Tripodis Y, Xia W et al (2018) Lewy body pathology and chronic traumatic encephalopathy associated with contact sports. J Neuropathol Exp Neurol 77:757–768. https://doi.org/10.1093/jnen/nly065
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E et al (2014) Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 83:406–412. https://doi.org/10.1212/WNL.0000000000000641
Alosco ML, Stein TD, Tripodis Y, Chua AS, Kowall NW, Huber BR et al (2019) Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol 02118:1298–1308. https://doi.org/10.1001/jamaneurol.2019.2244
Beach TG, Adler CH, Lue LF, Sue LI, Bachalakuri J, Henry-Watson J et al (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634. https://doi.org/10.1007/s00401-009-0538-8
Benarroch EE, Schmeichel AM, Sandroni P, Parisi JE, Low PA (2007) Rostral raphe involvement in Lewy body dementia and multiple system atrophy. Acta Neuropathol 114:213–220. https://doi.org/10.1007/s00401-007-0260-3
Bieniek KF, Blessing MM, Heckman MG, Diehl NN, Serie AM, Paolini MA et al (2020) Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol 30:63–74. https://doi.org/10.1111/bpa.12757
Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE et al (2015) Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 130:877–889. https://doi.org/10.1007/s00401-015-1502-4
Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM et al (2016) Traumatic brain injuries. Nat Rev Dis Prim 2:16084. https://doi.org/10.1038/nrdp.2016.84
Boeve BF (2010) REM sleep behavior disorder updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 1184:15–54
Boeve BF (2013) Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol 12:469–482. https://doi.org/10.1016/S1474-4422(13)70054-1
Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M et al (2013) Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 9:475–480. https://doi.org/10.5664/jcsm.2670
Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K et al (2011) Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 12:445–453. https://doi.org/10.1016/j.sleep.2010.12.009
Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM et al (2013) Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 14:754–762. https://doi.org/10.1016/j.sleep.2012.10.015
Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE (2001) Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 16:622–630. https://doi.org/10.1002/mds.1120
Boeve BF, Silber MH, Parisi JE, Dickson DW, Ferman TJ, Benarroch EE et al (2003) Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 61:40–45. https://doi.org/10.1212/01.WNL.0000073619.94467.B0
Boeve BF, Silber MH, Saper CB, Ferman J, Dickson DW, Parisi JE et al (2007) Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130:2770–2788. https://doi.org/10.1093/brain/awm056
Boot BP, Boeve BF, Roberts RO, Ferman TJ, Geda YE, Pankratz VS et al (2012) Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol 71:49–56. https://doi.org/10.1002/ana.22655
Campbell G, Pennello G, Yue L (2011) Missing data in the regulation of medical devices. J Biopharm Stat 21:180–195. https://doi.org/10.1080/10543406.2011.550094
Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Dirk Keene C et al (2016) Association of traumatic brain injury with late-life neurodegenerative conditions and Neuropathologic findings. JAMA Neurol 73:1062–1069. https://doi.org/10.1001/jamaneurol.2016.1948
Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi P, Plazzi G et al (2018) REM sleep behaviour disorder. Nat Rev Dis Prim. https://doi.org/10.1038/s41572-018-0016-5
Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O et al (2011) Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 77:875–882. https://doi.org/10.1212/WNL.0b013e31822c9148
Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K (2015) Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 77:987–995. https://doi.org/10.1002/ana.24396
Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K (2018) Mild TBI and risk of parkinson disease: a chronic effects of neurotrauma consortium study. Neurology 90:e1771–e1779. https://doi.org/10.1212/WNL.0000000000005522
Goldstein LE, Fisher AM, Tagge CA, Zhang X, Velisek L, Sullivan JA et al (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4:293–299. https://doi.org/10.1126/scitranslmed.3003716.Chronic
Haba-Rubio J, Frauscher B, Marques-Vidal P, Toriel J, Tobback N, Andries D et al (2018) Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 41:1–8. https://doi.org/10.1093/sleep/zsx197
Halliday GM, Blumbergs PC, Cotton RGH, Blessing WW, Geffen LB (1990) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107. https://doi.org/10.1016/0006-8993(90)90733-R
Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RGH, Howe PRC et al (1990) Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 27:373–385. https://doi.org/10.1002/ana.410270405
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Iranzo A, Santamaria J (2005) Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep 28:203–206. https://doi.org/10.1093/sleep/28.2.203
Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M et al (2013) Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 12:443–453. https://doi.org/10.1016/S1474-4422(13)70056-5
Jafari S, Etminan M, Aminzadeh F, Samii A (2013) Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 28:1222–1229. https://doi.org/10.1002/mds.25458
Jellinger KA (1991) Pathology of Parkinson’s disease: changes other than the nigrostriatal pathway. In: Molecular and chemical neuropathology. pp 153–197
Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S, Waki R (2000) A discrete pontine ischemic lesion could cause REM sleep behavior disorder. Neurology 55:894–895
King A, Sweeney F, Bodi I, Troakes C, Maekawa S, Al-Sarraj S (2010) Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology 30:408–419. https://doi.org/10.1111/j.1440-1789.2009.01085.x
Koga S, Aoki N, Uitti RJ, Van Gerpen JA, Cheshire WP, Josephs KA et al (2015) When DLB, PD, and PSP masquerade as MSA. Neurology 85:404–412. https://doi.org/10.1212/WNL.0000000000001807
Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H et al (2016) Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131:87–102. https://doi.org/10.1007/s00401-015-1509-x
Krohn L, Wu RYJ, Heilbron K, Ruskey JA, Laurent SB, Blauwendraat C et al (2020) Fine-mapping of SNCA in rapid eye movement sleep behavior disorder and overt synucleinopathies. Ann Neurol 87:584–598. https://doi.org/10.1002/ana.25687
Lehman EJ, Hein MJ, Baron SL, Gersic CM (2012) Neurodegenerative causes of death among retired national football league players. Neurology 79:1970–1974. https://doi.org/10.1212/WNL.0b013e31826daf50
Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL et al (2017) Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 133:337–352. https://doi.org/10.1007/s00401-017-1680-3
St. Louis EK, McCarter SJ, Boeve BF, Silber MH, Kantarci K, Benarroch EE et al (2014) Lesional REM sleep behavior disorder localizes to the dorsomedial pons. Neurology 83:1871–1873
Lu J, Sherman D, Devor M, Saper CB (2006) A putative flip-flop switch for control of REM sleep. Nature 441:589–594. https://doi.org/10.1038/nature04767
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Dirk Keene C, Litvan I et al (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131:75–86. https://doi.org/10.1007/s00401-015-1515-z
Mckee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW et al (2010) TDP-43 proteinopathy and motor neuron disease in CTE. J Neuropathol Exp Neurol 69:918–929. https://doi.org/10.1097/NEN.0b013e3181ee7d85.TDP-43
McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. https://doi.org/10.1093/brain/aws307
McKenna D, Peever J (2017) Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord 32:636–644. https://doi.org/10.1002/mds.27003
Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT et al (2020) Duration of American football play and chronic traumatic encephalopathy. Ann Neurol 87:116–131. https://doi.org/10.1002/ana.25611
Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR et al (2017) Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318:360–370. https://doi.org/10.1001/jama.2017.8334
Mez J, Solomon TM, Daneshvar DH, Murphy L, Kiernan PT, Montenigro PH et al (2015) Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimer’s Res Ther 7:1–14. https://doi.org/10.1186/s13195-015-0148-8
Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R et al (2014) Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer’s Res Ther 6:68. https://doi.org/10.1186/s13195-014-0068-z
Monti JM (2010) The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev 14:319–327. https://doi.org/10.1016/j.smrv.2009.10.003
Monti JM (2011) Serotonin control of sleep-wake behavior. Sleep Med Rev 15:269–281. https://doi.org/10.1016/j.smrv.2010.11.003
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Nitz D, Siegel J (1997) GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol 273:451–455. https://doi.org/10.1152/ajpregu.1997.273.1.R451
Paulus W, Jellinger KA (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50:743–755
Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E et al (2015) REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov Disord 30:1413–1417. https://doi.org/10.1002/mds.26347
Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y et al (2012) A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord 27:913–916. https://doi.org/10.1002/mds.25037
Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S et al (2012) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord 27:720–726. https://doi.org/10.1002/mds.24939
Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY (2012) How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 135:1860–1870. https://doi.org/10.1093/brain/aws093
Postuma RB, Montplaisir JY, Pelletier A, Dauvilliers Y, Oertel W, Iranzo A et al (2012) Environmental risk factors for REM sleep behavior disorder. Neurology 79:248–434
Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW (1986) Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep 9:293–308. https://doi.org/10.1093/sleep/9.2.293
Schenck CH, Bundlie SR, Patterson AL, Mahowald MW (1987) Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA 257:1786–1789. https://doi.org/10.1001/jama.1987.03390130104038
Shprecher DR, Adler CH, Zhang N, Hentz JG, Serrano GE, Dugger BN et al (2018) Predicting alpha-synuclein pathology by REM sleep behavior disorder diagnosis. Park Relat Disord 55:92–96. https://doi.org/10.1016/j.parkreldis.2018.05.020
Siegel JM (2017) Rapid eye movement sleep. In: Kryger M, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 6th edn. Elsevier Inc, Philadelphia, pp 78–95
St Louis EK, Boeve AR, Boeve BF (2017) REM sleep behavior disorder in Parkinson’s disease and other synucleinopathies. Mov Disord 32:645–658. https://doi.org/10.1002/mds.27018
Standring OJ, Friedberg J, Tripodis Y, Chua AS, Cherry JD, Alvarez VE et al (2019) Contact sport participation and chronic traumatic encephalopathy are associated with altered severity and distribution of cerebral amyloid angiopathy. Acta Neuropathol 138:401–413. https://doi.org/10.1007/s00401-019-02031-x
Stein TD, Alvarez VE, McKee AC (2015) Concussion in chronic traumatic encephalopathy. Curr Pain Headache Rep 19:2–7. https://doi.org/10.1007/s11916-015-0522-z
Stein TD, Montenigro PH, Alvarez VE, Xia W, Crary JF, Tripodis Y et al (2015) Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 130:21–34. https://doi.org/10.1007/s00401-015-1435-y
Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO et al (2013) Clinical presentation of chronic traumatic encephalopathy. Neurology 81:1122–1129. https://doi.org/10.1212/WNL.0b013e3182a55f7f
Tippmann-Peikert M, Boeve BF, Keegan BM (2006) REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurology 66:1277–1278
Ursin R (2008) Changing concepts on the role of serotonin in the regulation of sleep and waking. In: Serotonin and Sleep: molecular, functional and clinical aspects. pp 3–21
Valencia Garcia S, Brischoux F, Clément O, Libourel PA, Arthaud S, Lazarus M et al (2018) Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat Commun 9:1–11. https://doi.org/10.1038/s41467-017-02761-0
Vonsattel JPG, del Amaya MP, Keller CE (2008) Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol 115:509–532. https://doi.org/10.1007/s00401-007-0311-9
Acknowledgements
We gratefully acknowledge the use of resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA) as well as all the individuals whose participation and contributions made this work possible.
Funding
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development Merit Award (I01-CX001038); Veterans Affairs Biorepository (BX002466); Alzheimer’s Association (NIRG-305779, NIRG-362697); National Institute of General Medical Sciences (5T32GM007198); National Institute of Aging (RF1AG054156, R56AG057768, RF1AG057768, K23AG046377); National Institute of Neurological Disorders and Stroke (U54NS115266, U01NS086659, K23NS102399); National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345-5); Department of Defense Peer Reviewed Alzheimer’s Research Program (PRARP #13267017); and the Concussion Legacy Foundation. This work was also supported by unrestricted gifts from the Andlinger Foundation and WWE.
Author information
Authors and Affiliations
Contributions
Study conception and design was led by JWA, MLA, JM, and TDS. The acquisition, analysis, or interpretation of data was performed by JWA, MLA, JM, VEA, BRH, YT, CHA, CK, KAC, RM, RN, HJK, NS, MU, EN, NA, JDC, CJN, NWK, LEG, BD, DIK, RCC, RAS, ACM, and TDS. The manuscript was drafted by JWA, MLA, JM, and TDS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Michael L. Alosco has received honorarium as a Scientific Advisor for Corino Therapeutics, Inc. Dr Goldstein is a paid consultant to Johnson & Johnson, Janssen Research & Development LLC, and Rebiscan Inc, and has received funding from the WWE (World Wrestling Entertainment) and Ivivi Health Sciences. Dr Stern has received research funding from Avid Radiopharmaceuticals Inc, is a member of the Mackey-White Committee of the National Football League Players Association, is a paid consultant to Biogen and Eli Lilly, receives royalties for published neuropsychological tests from Psychological Assessment Resources Inc, and is a member of the Board of Directors of King-Devick Technologies. Dr Cantu is a paid consultant to the National Football League Head Neck and Spine Committee, a vice president and chair of the scientific advisory committee of the National Operating Committee on Standards for Athletic Equipment, and a consultant to the Concussion Legacy Foundation; he also receives royalties from Houghton Mifflin Harcourt and compensation for expert legal opinion to the National Collegiate Athletic Association and National Hockey League and is a member of the Mackey-White Committee of the National Football League Players Association. Dr McKee is a member of the Mackey-White Committee of the National Football League Players Association and reports receiving grants from the National Institutes of Health and Department of Veteran Affairs. Dr Alosco reported grants from National Institutes of Health/National Institute of Neurological Disorders and Stroke during the conduct of the study. Dr Katz reported grants from Boston University School of Medicine Department of Neurology during the conduct of the study. Dr Stern reported grants from the National Institutes of Health during the conduct of the study; personal fees from Biogen and Eli Lilly outside the submitted work; membership on the board of directors for King-Devick Technologies, with stock options; and royalties for published neuropsychological tests from Psychological Assessment Resources Inc. Dr Mez reported grants from the National Institutes of Health, Department of Defense, Alzheimer’s Association, and Concussion Legacy Foundation during the conduct of the study. No other disclosures were reported.
Ethics approval
The study was performed in accordance with the ethical standards established by the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Institutional review board approval was granted from the Boston University Medical Center (BUMC) and the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA. The BUMC IRB also granted approval for postmortem clinical record review, neuropathological evaluation, and clinical interviews with donor family members.
Consent to participate
Written consent for brain donation and research participation was provided by donor next-of-kin.
Consent to publish
Not applicable.
Disclaimer
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Veterans Affairs or Department of Defense position, policy or decision, unless so designated by other official documentation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adams, J.W., Alosco, M.L., Mez, J. et al. Association of probable REM sleep behavior disorder with pathology and years of contact sports play in chronic traumatic encephalopathy. Acta Neuropathol 140, 851–862 (2020). https://doi.org/10.1007/s00401-020-02206-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-020-02206-x