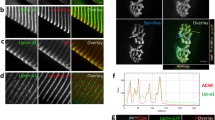
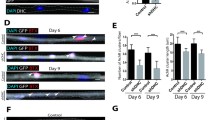
Abstract
The synaptic cleft of the neuromuscular junction (NMJ) consists of a highly specialized extracellular matrix (ECM) involved in synapse maturation, in the juxtaposition of pre- to post-synaptic areas, and in ensuring proper synaptic transmission. Key components of synaptic ECM, such as collagen IV, perlecan and biglycan, are binding partners of one of the most abundant ECM protein of skeletal muscle, collagen VI (ColVI), previously never linked to NMJ. Here, we demonstrate that ColVI is itself a component of this specialized ECM and that it is required for the structural and functional integrity of NMJs. In vivo, ColVI deficiency causes fragmentation of acetylcholine receptor (AChR) clusters, with abnormal expression of NMJ-enriched proteins and re-expression of fetal AChRγ subunit, both in Col6a1 null mice and in patients affected by Ullrich congenital muscular dystrophy (UCMD), the most severe form of ColVI-related myopathies. Ex vivo muscle preparations from ColVI null mice revealed altered neuromuscular transmission, with electrophysiological defects and decreased safety factor (i.e., the excess current generated in response to a nerve impulse over that required to reach the action potential threshold). Moreover, in vitro studies in differentiated C2C12 myotubes showed the ability of ColVI to induce AChR clustering and synaptic gene expression. These findings reveal a novel role for ColVI at the NMJ and point to the involvement of NMJ defects in the etiopathology of ColVI-related myopathies.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ackley BD, Kang SH, Crew JR, Suh C, Jin Y, Kramer JM (2003) The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J Neurosci 23:3577–3587. https://doi.org/10.1523/JNEUROSCI.23-09-03577.2003
Amenta AR, Creely HE, Mercado MLT, Hagiwara H, McKechnie BA, Lechner BE, Rossi SG, Wang Q, Owens RT, Marrero E, Mei L, Hoch W, Young MF, McQuillan DJ, Rotundo RL, Fallon JR (2012) Biglycan is an extracellular musk binding protein important for synapse stability. J Neurosci 32:2324–2334. https://doi.org/10.1523/jneurosci.4610-11.2012
Andonian MH, Fahim MA (1987) Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Neurocytol 16:589–599. https://doi.org/10.1007/BF01637652
Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y (2002) Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci 5:119–123. https://doi.org/10.1038/nn801
Biral D, Senter L, Salviati G (1996) Increased expression of dystrophin, β-dystroglycan and adhalin in denervated rat muscles. J Muscle Res Cell Motil 17:523–532. https://doi.org/10.1007/BF00124352
Blottner D, Lück G (2001) Just in time and place: NOS/NO system assembly in neuromuscular junction formation. Microsc Res Tech 55:171–180. https://doi.org/10.1002/jemt.1168
Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM (1998) Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet 7:2135–2140. https://doi.org/10.1093/hmg/7.13.2135
Bönnemann CG (2011) The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol 7:379–390. https://doi.org/10.1038/nrneurol.2011.81
Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M (2014) Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8:1509–1521. https://doi.org/10.1016/j.celrep.2014.07.061
Castagnaro S, Pellegrini C, Pellegrini M, Chrisam M, Sabatelli P, Toni S, Grumati P, Ripamonti C, Pratelli L, Maraldi NM, Cocchi D, Righi V, Faldini C, Sandri M, Bonaldo P, Merlini L (2016) Autophagy activation in COL6 myopathic patients by a low-protein-diet pilot trial. Autophagy 12:2484–2495. https://doi.org/10.1080/15548627.2016.1231279
Cescon M, Gattazzo F, Chen P, Bonaldo P (2015) Collagen VI at a glance. J Cell Sci 128:3525–3531. https://doi.org/10.1242/jcs.169748
Chang Y-F, Liu T-Y, Liu S-T (2013) Arecoline inhibits and destabilizes agrin-induced acetylcholine receptor cluster formation in C2C12 myotubes. Food Chem Toxicol 60:391–396. https://doi.org/10.1016/j.fct.2013.07.079
Chen P, Cescon M, Megighian A, Bonaldo P (2014) Collagen VI regulates peripheral nerve myelination and function. FASEB J 28:1145–1156. https://doi.org/10.1096/fj.13-239533
Cheusova T, Khan MA, Schubert SW, Gavin AC, Buchou T, Jacob G, Sticht H, Allende J, Boldyreff B, Brenner HR, Hashemolhosseini S (2006) Casein kinase 2-dependent serine phosphorylation of MuSK regulates acetylcholine receptor aggregation at the neuromuscular junction. Genes Dev 20:1800–1816. https://doi.org/10.1101/gad.375206
Chrisam M, Pirozzi M, Castagnaro S, Blaauw B, Polishchuck R, Cecconi F, Grumati P, Bonaldo P (2015) Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy 11:2142–2152. https://doi.org/10.1080/15548627.2015.1108508
de Kerchove d’Exaerde A, Cartaud J, Ravel-Chapuis A, Seroz T, Pasteau F, Angus LM, Jasmin BJ, Changeux J-P, Schaeffer L (2002) Expression of mutant Ets protein at the neuromuscular synapse causes alterations in morphology and gene expression. EMBO Rep 3:1075–1081. https://doi.org/10.1093/embo-reports/kvf220
Dorninger F, Herbst R, Kravic B, Camurdanoglu BZ, Macinkovic I, Zeitler G, Forss-Petter S, Strack S, Khan MM, Waterham HR, Rudolf R, Hashemolhosseini S, Berger J (2017) Reduced muscle strength in ether lipid-deficient mice is accompanied by altered development and function of the neuromuscular junction. J Neurochem. https://doi.org/10.1111/jnc.14082
Dowling JJ, Gonorazky HD, Cohn RD, Campbell C (2017) Treating pediatric neuromuscular disorders: the future is now. Am J Med Genet Part A. https://doi.org/10.1002/ajmg.a.38418
Eftimie R, Brenner HR, Buonanno A (1991) Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA 88:1349–1353. https://doi.org/10.1073/pnas.88.4.1349
Fox MA (2008) Novel roles for collagens in wiring the vertebrate nervous system. Curr Opin Cell Biol 20:508–513. https://doi.org/10.1016/j.ceb.2008.05.003
Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fässler R, Hudson BG, John SWM, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H (2007) Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129:179–193. https://doi.org/10.1016/j.cell.2007.02.035
Gara SK, Grumati P, Squarzoni S, Sabatelli P, Urciuolo A, Bonaldo P, Paulsson M, Wagener R (2011) Differential and restricted expression of novel collagen VI chains in mouse. Matrix Biol 30:248–257. https://doi.org/10.1016/j.matbio.2011.03.006
Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M, Paulsson M, Wagener R (2008) Three novel collagen VI chains with high homology to the α3 chain. J Biol Chem 283:10658–10670. https://doi.org/10.1074/jbc.M709540200
Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85:525–535. https://doi.org/10.1016/S0092-8674(00)81253-2
Gramolini AO, Burton EA, Tinsley JM, Ferns MJ, Cartaud A, Cartaud J, Davies KE, Lunde JA, Jasmin BJ (1998) Muscle and neural isoforms of agrin increase utrophin expression in cultured myotubes via a transcriptional regulatory mechanism. J Biol Chem 273:736–743. https://doi.org/10.1074/jbc.273.2.736
Gramolini AO, Jasmin BJ (1999) Expression of the utrophin gene during myogenic differentiation. Nucleic Acids Res 27:3603–3609. https://doi.org/10.1093/nar/27.17.3603
Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P (2010) Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16:1313–1320. https://doi.org/10.1038/nm.2247
Grumati P, Coletto L, Schiavinato A, Castagnaro S, Bertaggia E, Sandri M, Bonaldo P (2011) Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 7:1415–1423. https://doi.org/10.4161/auto.7.12.17877
Hunter JM, Ellen Ahearn M, Balak CD, Liang WS, Kurdoglu A, Corneveaux JJ, Russell M, Huentelman MJ, Craig DW, Carpten J, Coons SW, DeMello DE, Hall JG, Bernes SM, Baumbach-Reardon L, Jesse Hunter CM, Clow K (2015) Novel pathogenic variants and genes for myopathies identified by whole exome sequencing. Mol Genet Genom Med 3:283–301. https://doi.org/10.1002/mgg3.142
Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P (2003) Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet 35:367–371. https://doi.org/10.1038/ng1270
Kuo H-J, Maslen CL, Keene DR, Glanville RW (1997) Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem 272:26522–26529. https://doi.org/10.1074/jbc.272.42.26522
Lacazette E, Le Calvez S, Gajendran N, Brenner HR (2003) A novel pathway for MuSK to induce key genes in neuromuscular synapse formation. J Cell Biol 161:727–736. https://doi.org/10.1083/jcb.200210156
Liley AW (1956) The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol 134:427–443. https://doi.org/10.1113/jphysiol.1956.sp005655
Macpherson PCD, Cieslak D, Goldman D (2006) Myogenin-dependent nAChR clustering in aneural myotubes. Mol Cell Neurosci 31:649–660. https://doi.org/10.1016/j.mcn.2005.12.005
Massoulié J, Millard CB (2009) Cholinesterases and the basal lamina at vertebrate neuromuscular junctions. Curr Opin Pharmacol 9:316–325. https://doi.org/10.1016/j.coph.2009.04.004
Meier T, Masciulli F, Moore C, Schoumacher F, Eppenberger U, Denzer AJ, Jones G, Brenner HR (1998) Agrin can mediate acetylcholine receptor gene expression in muscle by aggregation of muscle-derived neuregulins. J Cell Biol 141:715–726. https://doi.org/10.1083/jcb.141.3.715
Méjat A, Decostre V, Li J, Renou L, Kesari A, Hantaï D, Stewart CL, Xiao X, Hoffman E, Bonne G, Misteli T (2009) Lamin A/C-mediated neuromuscular junction defects in Emery–Dreifuss muscular dystrophy. J Cell Biol 184:31–44. https://doi.org/10.1083/jcb.200811035
Merlini L, Martoni E, Grumati P, Sabatelli P, Squarzoni S, Urciuolo A, Ferlini A, Gualandi F, Bonaldo P (2008) Autosomal recessive myosclerosis myopathy is a collagen VI disorder. Neurology 71:1245–1253. https://doi.org/10.1212/01.wnl.0000327611.01687.5e
Miner JH (1994) Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127:879–891. https://doi.org/10.1083/jcb.127.3.879
Mongiovi PC, Elsheikh B, Lawson VH, Kissel JT, Arnold WD (2014) Neuromuscular junction disorders mimicking myopathy. Muscle Nerve 50:854–856. https://doi.org/10.1002/mus.24300
Nishimune H, Valdez G, Jarad G, Moulson CL, Müller U, Miner JH, Sanes JR (2008) Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol 182:1201–1215. https://doi.org/10.1083/jcb.200805095
Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP (1995) Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 374:258–262. https://doi.org/10.1038/374258a0
Ohno K, Sadeh M, Blatt I, Brengman JM, Engel AG (2003) E-box mutations in the RAPSN promoter regions in eight cases with congenital myasthenic syndrome. Hum Mol Genet 12:739–748. https://doi.org/10.1093/hmg/ddg089
Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edström L, Tryggvason K, Sanes JR (2001) Properly formed but improperly localized synaptic specializations in the absence of laminin α4. Nat Neurosci 4:597–604. https://doi.org/10.1038/88414
Patton BL, Miner JH, Chiu AY, Sanes JR (1997) Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol 139:1507–1521. https://doi.org/10.1083/jcb.139.6.1507
Peng HB, Xie H, Rossi SG, Rotundo RL (1999) Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol 145:911–921. https://doi.org/10.1083/jcb.145.4.911
Plomp JJ, van Kempen GT, Molenaar PC (1992) Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol 458:487–499. https://doi.org/10.1113/jphysiol.1992.sp019429
Robinson KG, Mendonca JL, Militar JL, Theroux MC, Dabney KW, Shah SA, Miller F, Akins RE (2013) Disruption of basal lamina components in neuromotor synapses of children with spastic quadriplegic cerebral palsy. PLoS One 8:e70288. https://doi.org/10.1371/journal.pone.0070288
Rogozhin AA, Pang KK, Bukharaeva E, Young C, Slater CR (2008) Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol 586:3163–3182. https://doi.org/10.1113/jphysiol.2008.153569
Rudolf R, Khan MM, Labeit S, Deschenes MR (2014) Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00099
Sabatelli P, Bonaldo P, Lattanzi G, Braghetta P, Bergamin N, Capanni C, Mattioli E, Columbaro M, Ognibene A, Pepe G, Bertini E, Merlini L, Maraldi NM, Squarzoni S (2001) Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol 20:475–486. https://doi.org/10.1016/S0945-053X(01)00160-3
Sabatelli P, Gara SK, Grumati P, Urciuolo A, Gualandi F, Curci R, Squarzoni S, Zamparelli A, Martoni E, Merlini L, Paulsson M, Bonaldo P, Wagener R (2011) Expression of the collagen VI α5 and α6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J Invest Dermatol 131:99–107. https://doi.org/10.1038/jid.2010.284
Sabatelli P, Gualandi F, Gara SK, Grumati P, Zamparelli A, Martoni E, Pellegrini C, Merlini L, Ferlini A, Bonaldo P, Maraldi NM, Paulsson M, Squarzoni S, Wagener R (2012) Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol 31:187–196. https://doi.org/10.1016/j.matbio.2011.12.003
Sala C, Andreose JS, Fumagalli G, Lømo T (1995) Calcitonin gene-related peptide: possible role in formation and maintenance of neuromuscular junctions. J Neurosci 15:520–528. https://doi.org/10.1523/JNEUROSCI.15-01-00520.1995
Sandrock AW Jr (1997) Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science (80-) 276:599–603. https://doi.org/10.1126/science.276.5312.599
Sanes JR (2003) The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278:12601–12604. https://doi.org/10.1074/jbc.R200027200
Sarwal A, Walker FO, Cartwright MS (2013) Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 47:319–329. https://doi.org/10.1002/mus.23671
Schaeffer L, de Kerchove d’Exaerde A, Duclert N, Huchet-Dymanus M, Changeux J-P J-P (1998) Implication of a multisubunit Ets related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. J Physiol 92:489. https://doi.org/10.1016/S0928-4257(99)80106-5
Schessl J, Goemans NM, Magold AI, Zou Y, Hu Y, Kirschner J, Sciot R, Bönnemann CG (2008) Predominant fiber atrophy and fiber type disproportion in early Ullrich disease. Muscle Nerve 38:1184–1191. https://doi.org/10.1002/mus.21088
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531. https://doi.org/10.1152/physrev.00031.2010
Sigoillot SM, Bourgeois F, Karmouch J, Molgó J, Dobbertin A, Chevalier C, Houlgatte R, Léger J, Legay C (2016) Neuromuscular junction immaturity and muscle atrophy are hallmarks of the ColQ-deficient mouse, a model of congenital myasthenic syndrome with acetylcholinesterase deficiency. FASEB J 30:2382–2399. https://doi.org/10.1096/fj.201500162
Simon AM, Burden SJ (1993) An E box mediates activation and repression of the acetylcholine receptor delta-subunit gene during myogenesis. Mol Cell Biol 13:5133–5140. https://doi.org/10.1128/mcb.13.9.5133
Singhal N, Martin PT (2011) Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol 71:982–1005. https://doi.org/10.1002/dneu.20953
Tang H, Veldman MB, Goldman D (2006) Characterization of a muscle-specific enhancer in human MuSK promoter reveals the essential role of myogenin in controlling activity-dependent gene regulation. J Biol Chem 281:3943–3953. https://doi.org/10.1074/jbc.M511317200
Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF (1997) Expression of different isoforms of nitric oxide synthase in experimentally denervated and reinnervated skeletal muscle. J Neuropathol Exp Neurol 56:1283–1289. https://doi.org/10.1097/00005072-199712000-00003
Tillet E, Wiedemann H, Golbik R, Pan TC, Zhang RZ, Mann K, Chu ML, Timpl R (1994) Recombinant expression and structural and binding properties of alpha 1(VI) and alpha 2(VI) chains of human collagen type VI. Eur J Biochem 221:177–185. https://doi.org/10.1111/j.1432-1033.1994.tb18727.x
Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P (2013) Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4:1964. https://doi.org/10.1038/ncomms2964
Verma S, Goyal P, Guglani L, Peinhardt C, Pelzek D, Barkhaus PE (2018) COL6A and LAMA2 mutation congenital muscular dystrophy: a clinical and electrophysiological study. J Clin Neuromuscul Dis 30324:108–116. https://doi.org/10.1097/CND.0000000000000198
Wiberg C, Hedbom E, Khairullina A, Lamandé SR, Oldberg A, Timpl R, Mörgelin M, Heinegård D (2001) Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J Biol Chem 276:18947–18952. https://doi.org/10.1074/jbc.m100625200
Willadt S, Nash M, Slater CR (2016) Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci Rep 6:24849. https://doi.org/10.1038/srep24849
Witzemann V, Barg B, Criado M, Stein E, Sakmann B (1989) Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett 242:419–424. https://doi.org/10.1016/0014-5793(89)80514-9
Wood SJ, Slater CR (1997) The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fast-and slow-twitch muscles. J Physiol 500:65–176. https://doi.org/10.1113/jphysiol.1997.sp022007
Acknowledgements
We are grateful to R. Wagener for the 3(VI) antibody and Dr M. Vitadello for the neurofilament antibody. We also acknowledge support from Telethon Genetic BioBank (GTB12001D) and the Eurobiobank network. This work was supported by Italian Ministry of Education, University and Research (Grants RBAP11Z3YA_003 and 2015FBNB5Y), Telethon Foundation (Grant GGP14202) and University of Padova (to P.B.); Cariparo Foundation (Starting Grants 2015) (to M.C. and P.B.); German Research Council DFG (Grant HA3309/3-1) and Interdisciplinary Centre for Clinical Research at the University Hospital of the Friedrich-Alexander University of Erlangen-Nürnberg (Grant E17) (to S.H.). I.G. was supported by a PhD fellowship from the Cariparo Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cescon, M., Gregorio, I., Eiber, N. et al. Collagen VI is required for the structural and functional integrity of the neuromuscular junction. Acta Neuropathol 136, 483–499 (2018). https://doi.org/10.1007/s00401-018-1860-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1860-9