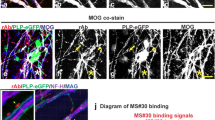
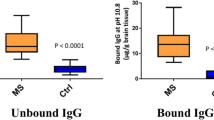
Abstract
B cells are implicated in the etiology of multiple sclerosis (MS). Intrathecal IgG synthesis, cerebrospinal fluid (CSF) oligoclonal bands and lesional IgG deposition suggest a role for antibody-mediated pathology. We examined the binding of IgG1 monoclonal recombinant antibodies (rAbs) derived from MS patient CSF expanded B cell clones to central nervous system (CNS) tissue. MS rAbs displaying CNS binding to mouse and human CNS tissue were further tested for their ability to induce complement-mediated tissue injury in ex vivo spinal cord explant cultures. The staining of CNS tissue, primary human astrocytes and human neurons revealed a measurable bias in MS rAb binding to antigens preferentially expressed on astrocytes and neurons. MS rAbs that recognize myelin-enriched antigens were rarely detected. Both myelin-specific and some astrocyte/neuronal-specific MS rAbs caused significant myelin loss and astrocyte activation when applied to spinal cord explant cultures in the presence of complement. Overall, the intrathecal B cell response in multiple sclerosis binds to both glial and neuronal targets and produces demyelination in spinal cord explant cultures implicating intrathecal IgG in MS pathogenesis.









Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, Oksenberg JR (1999) B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 163:5133–5144
Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209:1001–1010. doi:10.1084/jem.20111675
Bennett JL, Haubold K, Ritchie AM, Edwards SJ, Burgoon M, Shearer AJ, Gilden DH, Owens GP (2008) CSF IgG heavy-chain bias in patients at the time of a clinically isolated syndrome. J Neuroimmunol 199:126–132. doi:10.1016/j.jneuroim.2008.04.031
Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66:617–629. doi:10.1002/ana.21802
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. doi:10.1038/nature04753
Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, Adzemovic M, Bauer J, Berger T, Fujihara K, Itoyama Y, Lassmann H (2009) Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 66:630–643. doi:10.1002/ana.21837
Breij EC, Brink BP, Veerhuis R, van den Berg C, Vloet R, Yan R, Dijkstra CD, van der Valk P, Bo L (2008) Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol 63:16–25. doi:10.1002/ana.21311
Brennan KM, Galban-Horcajo F, Rinaldi S, O’Leary CP, Goodyear CS, Kalna G, Arthur A, Elliot C, Barnett S, Linington C, Bennett JL, Owens GP, Willison HJ (2011) Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J Neuroimmunol 238:87–95. doi:10.1016/j.jneuroim.2011.08.002
Burgoon MP, Hammack BN, Owens GP, Maybach AL, Eikelenboom MJ, Gilden DH (2003) Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol 54:459–463. doi:10.1002/ana.10685
Cepok S, Jacobsen M, Schock S, Omer B, Jaekel S, Boddeker I, Oertel WH, Sommer N, Hemmer B (2001) Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain J Neurol 124:2169–2176
Elliott C, Lindner M, Arthur A, Brennan K, Jarius S, Hussey J, Chan A, Stroet A, Olsson T, Willison H, Barnett SC, Meinl E, Linington C (2012) Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain J Neurol 135:1819–1833. doi:10.1093/brain/aws105
Gilden DH (2005) Infectious causes of multiple sclerosis. Lancet Neurol 4:195–202. doi:10.1016/S1474-4422(05)01017-3
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, Group HT (2008) B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 358:676–688. doi:10.1056/NEJMoa0706383
Henderson AP, Barnett MH, Parratt JD, Prineas JW (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753. doi:10.1002/ana.21800
Kabat EA, Moore DH, Landow H (1942) An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Investig 21:571–577. doi:10.1172/JCI101335
Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC (2006) The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain J Neurol 129:584–594. doi:10.1093/brain/awh721
Lisak RP, Benjamins JA, Nedelkoska L, Barger JL, Ragheb S, Fan B, Ouamara N, Johnson TA, Rajasekharan S, Bar-Or A (2012) Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J Neuroimmunol 246:85–95. doi:10.1016/j.jneuroim.2012.02.015
Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Obermeier B, Mentele R, Malotka J, Kellermann J, Kumpfel T, Wekerle H, Lottspeich F, Hohlfeld R, Dornmair K (2008) Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med 14:688–693. doi:10.1038/nm1714
Owens GP, Bennett JL (2012) Trigger, pathogen, or bystander: the complex nexus linking Epstein–Barr virus and multiple sclerosis. Mult Scler 18:1204–1208. doi:10.1177/1352458512448109
Owens GP, Bennett JL, Lassmann H, O’Connor KC, Ritchie AM, Shearer A, Lam C, Yu X, Birlea M, DuPree C, Williamson RA, Hafler DA, Burgoon MP, Gilden D (2009) Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol 65:639–649. doi:10.1002/ana.21641
Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, Gilden DH (1998) Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol 43:236–243. doi:10.1002/ana.410430214
Owens GP, Winges KM, Ritchie AM, Edwards S, Burgoon MP, Lehnhoff L, Nielsen K, Corboy J, Gilden DH, Bennett JL (2007) VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol 179:6343–6351
Prineas JW, Wright RG (1978) Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Investig J Tech Methods Pathol 38:409–421
Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2010) Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain J Neurol 133:349–361. doi:10.1093/brain/awp309
Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A, Bennett JL, Korn T, Hemmer B (2012) Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med 367:115–123. doi:10.1056/NEJMoa1110740
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285. doi:10.1056/NEJM199801293380502
Traugott U, Reinherz EL, Raine CS (1983) Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science 219:308–310
von Budingen HC, Harrer MD, Kuenzle S, Meier M, Goebels N (2008) Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol 38:2014–2023. doi:10.1002/eji.200737784
Winges KM, Gilden DH, Bennett JL, Yu X, Ritchie AM, Owens GP (2007) Analysis of multiple sclerosis cerebrospinal fluid reveals a continuum of clonally related antibody-secreting cells that are predominantly plasma blasts. J Neuroimmunol 192:226–234. doi:10.1016/j.jneuroim.2007.10.009
Yahr MD, Kabat EA (1957) Cerebrospinal fluid and serum gamma globulin levels in multiple sclerosis: changes induced by large doses of prednisone. Trans Am Neurol Assoc 115–118 (82nd Meeting; discussion 118–119)
Zhang H, Bennett JL, Verkman AS (2011) Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol 70:943–954. doi:10.1002/ana.22551
Acknowledgments
This work was supported by Public Health Service grants NS072141 (GPO) and EY022936 (JLB) awarded from the National Institutes of Health, and from support (JLB) provided by the Guthy-Jackson Foundation. K. B. Blauth was supported by training Grant NS007321 to D. H. Gilden from the National Institutes of Health. We kindly thank Dr. V. Wong, University of Calgary, Canada for providing human astrocyte cultures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2015_1500_MOESM2_ESM.jpg
Supplemental Fig. 1. Superbiochip array slides were deparaffinized and immunostained as described in the methods section. rAb immunostaining is in green, DAPI is in blue. Scale bar = 100 µm. (a) MS04-2 # 30 peripheral reactivity was punctate and often associated with epithelia. (b) MS05-3 # 38 peripheral reactivity was punctate in esophagus and pancreas but more ubiquitously expressed in ileum and skin. (c) IC05-2 # 2 reactivity was largely absent in the periphery (JPEG 89 kb)
Rights and permissions
About this article
Cite this article
Blauth, K., Soltys, J., Matschulat, A. et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid cause demyelination of spinal cord explants. Acta Neuropathol 130, 765–781 (2015). https://doi.org/10.1007/s00401-015-1500-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1500-6