Abstract
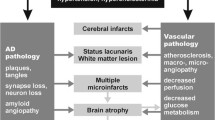
The progressive development of Alzheimer disease (AD)-related lesions, such as neurofibrillary tangles (NFT), amyloid deposits and synaptic loss, and the occurrence of microvascular and small macrovascular pathology within the cerebral cortex are conspicuous neuropathologic features of brain aging. Recent neuropathologic studies strongly suggested that the clinical diagnosis of dementia depends more on the severity and topography of pathological changes than on the presence of a qualitative marker. However, several methodological problems, such as selection biases, case–control design, density-based measures and masking effects, of concomitant pathologies persisted. In recent years, we performed several clinicopathologic studies using stereological counting of AD lesions. In order to define the cognitive impact of lacunes and microvascular lesions, we also analyzed pure vascular cases without substantial AD pathology. Our data revealed that total NFT numbers in the CA1 field, cortical microinfarcts and subcortical gray matter lacunes were the stronger determinants of dementia. In contrast, the contribution of periventricular and subcortical white matter demyelinations had a modest cognitive effect even in rare cases with isolated microvascular pathology. Importantly, in cases with pure AD pathology, more than 50% of Clinical Dementia Rating scale variability was not explained by NFT, amyloid deposits and neuronal loss in the hippocampal formation. In cases with microvascular pathology or lacunes, this percentage was even lower. The present review summarizes our data in this field and discusses their relevance within the theoretical framework of the functional neuropathology of brain aging and with particular reference to the current efforts to develop standardized neuropathological criteria for mixed dementia.



Similar content being viewed by others
References
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Berl) 37:111–118
Bancher C, Jellinger K, Lassmann H, Fischer P, Leblhuber F (1996) Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur Arch Psychiatry Clin Neurosci 246:137–146
Barcikowska M, Wisniewski HM, Bancher C, Grundke-Iqbal I (1989) About the presence of paired helical filaments in dystrophic neurites participating in the plaque formation. Acta Neuropathol (Berl) 78:225–231
Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66:1837–1844
Berg L, McKeel DW Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55:326–335
Bierer LM, Hof PR, Purohit KP, Carlin L, Schmeidler J, Davis KL, Perl DP (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52:81–88
Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 4:138–150
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153:131–135
Bouras C, Kövari E, Herrmann FR, Rivara CB, Bailey TL, von Gunten A, Hof PR, Giannakopoulos P (2006) Stereologic analysis of microvascular morphology in the elderly: Alzheimer disease pathology and cognitive status. J Neuropathol Exp Neurol 65:235–244
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 112:388–403
Braak H, Braak E (1997) Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging 18:S85–S88
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Braak E (1997) Staging of Alzheimer-related cortical destruction. Int Psychogeriatr 9(Suppl 1):257–261 (discussion 269–272)
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278 (discussion 278–284)
Braak H, Braak E, Bohl J (1993) Staging of Alzheimer-related cortical destruction. Eur Neurol 33:403–408
Bracco L, Campani D, Baratti E, Lippi A, Inzitari D, Pracucci G, Amaducci L (1993) Relation between MRI features and dementia in cerebrovascular disease patients with leukoaraiosis: a longitudinal study. J Neurol Sci 120:131–136
Bracco L, Piccini C, Moretti M, Mascalchi M, Sforza A, Nacmias B, Cellini E, Bagnoli S, Sorbi S (2005) Alzheimer’s disease: role of size and location of white matter changes in determining cognitive deficits. Dement Geriatr Cogn Disord 20:358–366
Bussière T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Giannakopoulos P, Robakis NK, Morrison JH, Perl DP, Hof PR (2002) Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience 112:75–91
Callahan LM, Vaules WA, Coleman PD (2002) Progressive reduction of synaptophysin message in single neurons in Alzheimer disease. J Neuropathol Exp Neurol 61:384–395
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L (1988) Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38:1682–1687
Cummings BJ, Pike CJ, Shankle R, Cotman CW (1996) Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiol Aging 17:921–933
Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50:873–880
Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376–388
Del Ser T, Bermejo F, Portera A, Arredondo JM, Bouras C, Constantinidis J (1990) Vascular dementia. A clinicopathological study. J Neurol Sci 96:1–17
Del Ser T, Hachinski V, Merskey H, Munoz DG (2005) Alzheimer’s disease with and without cerebral infarcts. J Neurol Sci 231:3–11
Delaere P, He Y, Fayet G, Duyckaerts C, Hauw JJ (1993) Beta A4 deposits are constant in the brain of the oldest old: an immunocytochemical study of 20 French centenarians. Neurobiol Aging 14:191–194
Derouesne C, Poirier J (1999) [Cerebral lacunae: still under debate]. Rev Neurol (Paris) 155:823–831
Desmond DW, Moroney JT, Sano M, Stern Y (2002) Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke 33:2254–2260
Dickson DW (1997) Neuropathological diagnosis of Alzheimer’s disease: a perspective from longitudinal clinicopathological studies. Neurobiol Aging 18:S21–S26
Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P (1995) Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 16:285–298 (discussion 298–304)
Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13:179–189
Duyckaerts C, Bennecib M, Grignon Y, Uchihara T, He Y, Piette F, Hauw JJ (1997) Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging 18:267–273
Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A (1988) Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry 51:1037–1044
Esiri MM (2000) Which vascular lesions are of importance in vascular dementia? Ann N Y Acad Sci 903:239–243
Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD (1999) Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease [letter]. Lancet 354:919–920
Esiri MM, Wilcock GK, Morris JH (1997) Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 63:749–753
Farkas E, de Vos RA, Donka G, Jansen Steur EN, Mihaly A, Luiten PG (2006) Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol (Berl) 111:150–157
Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64:575–611
Fernando MS, Ince PG (2004) Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 226:13–17
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB (2000) Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356:628–634
Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60:1495–1500
Giannakopoulos P, Hof PR, Giannakopoulos AS, Buée-Scherrer V, Surini M, Delacourte A, Bouras C (1994) Dementia in the oldest–old: quantitative analysis of 12 cases from a psychiatric hospital. Dementia 5:348–356
Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, Bouras C (1995) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol 52:1150–1159
Giannakopoulos P, Hof PR, Kövari E, Vallet PG, Bouras C (1996) Distinct patterns of neuronal loss and Alzheimer’s disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol 55:1210–1220
Gold G, Kövari E, Corte G, Herrmann FR, Canuto A, Bussière T, Hof PR, Bouras C, Giannakopoulos P (2001) Clinical validity of a beta-protein deposition staging in brain aging and Alzheimer disease. J Neuropathol Exp Neurol 60:946–952
Gold G, Kövari E, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P (2005) Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke 36:1184–1188
Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41:17–24
Gómez-Isla T, Price JL, McKeel Jr DW, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500
Green MS, Kaye JA, Ball MJ (2000) The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology 54:105–113
Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB (1999) Memory and mental status correlates of modified Braak staging. Neurobiol Aging 20:573–579
Harding AJ, Halliday GM, Kril JJ (1998) Variation in hippocampal neuron number with age and brain volume. Cereb Cortex 8:710–718
Hof PR, Bierer LM, Perl DP, Delacourte A, Buée L, Bouras C, Morrison JH (1992) Evidence for early vulnerability of the medial and inferior aspects of the temporal lobe in an 82 year old patient with preclinical signs of dementia: regional and laminar distribution of neurofibrillary tangles and senile plaques. Arch Neurol 49:946–953
Hof PR, Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH (2003) Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol 62:55–67
Hubbard BM, Fenton GW, Anderson JM (1990) A quantitative histological study of early clinical and preclinical Alzheimer’s disease. Neuropathol Appl Neurobiol 16:111–121
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM (1998) Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 57:1168–1174
Itoh Y, Yamada M, Suematsu N, Matsushita M, Otomo E (1998) An immunohistochemical study of centenarian brains: a comparison. J Neurol Sci 157:73–81
Jellinger KA (2005) Understanding the pathology of vascular cognitive impairment. J Neurol Sci 229–230:57–63
Jellinger KA, Attems J (2003) Incidence of cerebrovascular lesions in Alzheimer’s disease: a postmortem study. Acta Neuropathol (Berl) 105:14–17
Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A (1988) Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23:138–144
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ (2001) Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol 49:202–213
Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P (2004) Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke 35:410–414
Kril JJ, Patel S, Harding AJ, Halliday GM (2002) Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol (Berl) 103:370–376
Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ (2000) Small concomitant vascular lesions do not influence rates of cognitive decline in patients with alzheimer disease. Arch Neurol 57:1474–1479
Lewis DA, Campbell MJ, Terry RD, Morrison JH (1987) Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci 7:1799–1808
Leys D, Scheltens P, Vermersch P, Pruvo JP (1995) [Morphological imaging in the diagnosis of dementia. II. Vascular dementia]. Rev Med Interne 16:195–200
Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 27:1274–1282
Mackowiak-Cordoliani MA, Bombois S, Memin A, Henon H, Pasquier F (2005) Poststroke dementia in the elderly. Drugs Aging 22:483–493
Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD (1993) Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43:192–197
Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46:707–719
Mountjoy CQ, Roth M, Evans NJ, Evans HM (1983) Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging 4:1–11
Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56:165–170
Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283:1571–1577
Neuropathology Group of the Medical Research Council Cognitive Function, Ageing Study (MRC CFAS) (2001) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 357:169–175
Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Price JL, Davis PB, Morris JC, White DL (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging 12:295–312
Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC (2001) Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 58:1395–1402
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Reed BR, Eberling JL, Mungas D, Weiner M, Jagust WJ (2001) Frontal lobe hypometabolism predicts cognitive decline in patients with lacunar infarcts. Arch Neurol 58:493–497
Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR (2005) Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging 26:341–347
Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC (2002) Subcortical ischaemic vascular dementia. Lancet Neurol 1:426–436
Samuel W, Masliah E, Hill LR, Butters N, Terry R (1994) Hippocampal connectivity and Alzheimer’s dementia: effects of synapse loss and tangle frequency in a two-component model. Neurology 44:2081–2088
Schmidt R, Schmidt H, Kapeller P, Lechner A, Fazekas F (2002) Evolution of white matter lesions. Cerebrovasc Dis 13:16–20
Schroeter ML, Bucheler MM, Preul C, Scheid R, Schmiedel O, Guthke T, von Cramon DY (2005) Spontaneous slow hemodynamic oscillations are impaired in cerebral microangiopathy. J Cereb Blood Flow Metab 25:1675–1684
Simic G, Kostovic I, Winblad B, Bogdanovic N (1997) Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol 379:482–494
Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR (1997) Brain infarction and the clinical expression of Alzheimer disease. The nun study. JAMA 277:813–817
Tatemichi TK, Paik M, Bagiella E, Desmond DW, Stern Y, Sano M, Hauser WA, Mayeux R (1994) Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology 44:1885–1891
Terry RD, Masliah E, Hansen LA (1994) Structural basis of the cognitive alterations in Alzheimer’s disease. In: Terry RD, Katzman R, Bick KL (eds) Alzheimer’s disease. Raven Press, New York, pp 179–196
Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H (2000) Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–748
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–356
Udaka F, Sawada H, Kameyama M (2002) White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci 977:411–415
Ulrich J (1985) Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer’s disease and senile dementia of Alzheimer type. Ann Neurol 17:273–277
Uspenskaia O, Liebetrau M, Herms J, Danek A, Hamann GF (2004) Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci 5:37
van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P (2005) Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 36:2116–2120
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM (2003) Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348:1215–1222
Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC (2000) Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol 59:931–945
von Gunten A, Kövari E, Bussière T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos P (2006) Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging 27:270–277
von Gunten A, Kövari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P (2005) Stereologic analysis of hippocampal Alzheimer’s disease pathology in the oldest–old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol 193:198–206
Wen HM, Mok VC, Fan YH, Lam WW, Tang WK, Wong A, Huang RX, Wong KS (2004) Effect of white matter changes on cognitive impairment in patients with lacunar infarcts. Stroke 35:1826–1830
West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14:287–293
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344:769–772
West MJ, Gundersen HJ (1990) Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol 296:1–22
Wilcock GK, Esiri MM (1982) Plaques, tangles and dementia. A quantitative study. J Neurol Sci 56:343–356
Wisniewski HM, Silverman W (1997) Diagnostic criteria for the neuropathological assessment of Alzheimer’s disease: current status and major issues. Neurobiol Aging 18:S43–S50
Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R (1995) White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 26:1171–1177
Zekry D, Duyckaerts C, Moulias R, Belmin J, Geoffre C, Herrmann F, Hauw JJ (2002) Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 103:481–487
Acknowledgments
We thank Dr. C.B. Rivara, Dr. A.B. Rocher, Dr. F.R. Herrmann and Dr. T. Bussière for their participation in some of the studies discussed in this review. B. Wicinski, P. Lovero and M. Demiri provided excellent technical help. This work was supported by NIH grants AG02219 and AG05138 (PRH), and the Jérôme Tissières Foundation (PG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giannakopoulos, P., Gold, G., Kövari, E. et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 113, 1–12 (2007). https://doi.org/10.1007/s00401-006-0144-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0144-y