Abstract
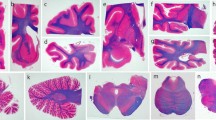
Alcoholic cerebellar degeneration (ACD) is a pivotal neurological complication in alcoholics. However, although there are a few autopsy reports and some data on its frequency, it is considered very rare in Japan. The aims of this study were (1) to estimate the frequency of the disease in Japanese autopsy cases, and (2) to examine the clinicopathological features of symptomatic and asymptomatic cases of ACD. We reviewed the records of 1,509 Japanese autopsies obtained from three autopsy series in Japan, and selected all 55 cases (3.6%) with alcoholism. On neuropathological reexamination, ACD was confirmed in six male alcoholics [0.4% of all subjects; 10.9% of all alcoholics; mean age at death 59.3±13.4 years (± SD)], including three asymptomatic cases. These frequencies were much lower than some previous Western findings, but more common than that has been expected in Japan. The frequencies of memory impairment and ataxia in ACD cases were significantly higher than those in alcoholics without any alcohol-related pathologies. In ACD cases, loss of Purkinje cells, narrowing of the width of the molecular layer, and tissue rarefaction in the granular layer were observed in the anterior and superior portions of the vermis of the cerebellum. In adjacent regions, the Purkinje cell and molecular layers were more mildly affected. The distribution of severely affected regions was more restricted in the asymptomatic cases than in the symptomatic cases. This study confirmed the frequency of asymptomatic cerebellar degeneration in alcoholics, suggesting that early intervention in alcoholism in the subclinical phase is important to prevent the development of cerebellar symptoms.

Similar content being viewed by others
References
Akai J, Akai K, Arai K (1987) Alcoholic cerebellar degeneration: a case study. Rinsho Shinkeigaku 27:1480–1485
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn (DSM-IV). American Psychiatric Association, Washington DC
Ando S, Murakami K (1985) Cerebellar degeneration in chronic alcoholism: with special reference to an autopsied case showing the restricted form of cerebellar cortical degeneration. No To Shinkei 37:329–336
Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG (1999) Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91:429–438
Caine D, Halliday GM, Kril JJ, Harper CG (1997) Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry 62:51–60
Estrin WJ (1987) Alcoholic cerebellar degeneration is not a dose-dependent phenomenon. Alcohol Clin Exp Res 11:372–375
Glanz J, Grant B, Monteiro M, Tabakoff B; WHO/ISBRA study on state and trait markers of alcohol use and dependence investigators (2002) WHO/ISBRA study on state and trait markers of alcohol use and dependence: analysis of demographic, behavioral, physiologic, and drinking variables that contribute to dependence and seeking treatment. International Society on Biomedical Research on Alcoholism. Alcohol Clin Exp Res 26: 1047–1061
Harper C (1979) Wernicke’s encephalopathy: a more common disease than realized. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry 42:226–231
Harper C, Dixon G, Sheedy D, Garrick T (2003) Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry 27:951–961
Helzer JE, Canino GJ, Yeh EK, Bland RC, Lee CK, Hwu HG, Newman S (1990) Alcoholism–North America and Asia. A comparison of population surveys with the diagnostic interview schedule. Arch Gen Psychiatry 47:313–319
Hillbom M, Muuronen A, Holm L, Hindmarsh T (1986) The clinical versus radiological diagnosis of alcoholic cerebellar degeneration. J Neurol Sci 73:45–53
Ishii N, Nishihara Y, Horie A (1980) Clinico-pathological studies on 62 autopsy cases of chronic alcoholics. Seishin Igaku (Clinical Psychiatry) 22:639–646
Iwabuchi K, Yagishita S, Itoh Y, Amano N, Saitoh A (1990) An autopsied case of alcoholic cerebellar degeneration with spastic paraplegia and neuropathy. No To Shinkei 42:489–496
Kawakami N, Shimizu H, Haratani T, Iwata N, Kitamura T (2004) Lifetime and 6-month prevalence of DSM-III-R psychiatric disorders in an urban community in Japan. Psychiatry Res 121:293–301
Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19
Kril JJ, Butterworth RF (1997) Diencephalic and cerebellar pathology in alcoholic and nonalcoholic patients with end-stage liver disease. Hepatology 26:837–841
Kromhout D, Bloemberg BP, Feskens EJ, Hertog MG, Menotti A, Blackburn H (1996) Alcohol, fish, fibre and antioxidant vitamins intake do not explain population differences in coronary heart disease mortality. Int J Epidemiol 25:753–759
Lindboe CF, Loberg EM (1988) The frequency of brain lesions in alcoholics. Comparison between the 5-year periods 1975–1979 and 1983–1987. J Neurol Sci 88:107–113
Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K (1999) Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cortex 9:712–721
Maschke M, Weber J, Bonnet U, Dimitrova A, Bohrenkamper J, Sturm S, Muller BW, Gastpar M, Diener HC, Forsting M, Timmann D (2005) Vermal atrophy of alcoholics correlate with serum thiamine levels but not with dentate iron concentrations as estimated by MRI. J Neurol 252:704–711
Matsushita M, Hanawa S (1983) An autopsied case with alcoholic cerebellar degeneration. Seishin Shinkeigaku Zasshi 85:542–560
Melgaard B, Ahlgren P (1986) Ataxia and cerebellar atrophy in chronic alcoholics. J Neurol 233:13–15
Mizutani T, Maeda S, Hayakawa K, Tanaka U, Hirahata S, Kamoshita H, Taketani T, Morimatsu Y (1988) Paraneoplastic cortical cerebellar degeneration. A neuropathological study of an autopsy case in comparison with cortical cerebellar degeneration in alcoholics. Acta Neuropathol (Berl) 77:206–212
Nicolas JM, Fernandez-Sola J, Robert J, Antunez E, Cofan M, Cardenal C, Sacanella E, Estruch R, Urbano-Marquez A (2000) High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. QJM 93:449–456
Nukada A (1973) Urbanization and consumption of alcoholic beverages. J Hum Ergol (Tokyo) 1:29–44
Phillips SC, Harper CG, Kril J (1987) A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain 110:301–314
Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD (1998) Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol 19:65–71
Smith EM (1982) An analysis of cohort mortality from tongue cancer in Japan, England and Wales and the United States. Int J Epidemiol 11:329–335
Stamler J, Elliott P, Chan Q for the INTERMAP Research Group (2003) INTERMAP Appendix Tables, Tables of Contents (Tables B). J Hum Hypertens 17:759–775
Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000) Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 14:341–352
Torvik A, Torp S (1986) The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. J Neurol Sci 75:43–51
Torvik A, Lindboe CF, Rogde S (1982) Brain lesions in alcoholics. A neuropathological study with clinical correlations. J Neurol Sci 56:233–248
Tsuchiya K, Watabiki S, Shiojiri T, Matsumoto A, Tsukagoshi H (1993) Alcoholic cerebellar degeneration with pyramidal sign–in relation to alcoholic myelopathy. No To Shinkei 45:169–175
Tsuchiya K, Ozawa E, Saito F, Irie H, Mizutani T (1994) Neuropathology of late cortical cerebellar atrophy in Japan: distribution of cerebellar change on an autopsy case and review of Japanese cases. Eur Neurol 34:253–262
Victor M, Laureno R (1978) Neurologic complications of alcohol abuse: epidemiologic aspects. Adv Neurol 19:603–617
Victor M, Adams RD, Mancall EL (1959) A restricted form of cerebellar degeneration occurring in alcoholic patients. Arch Neurol 1:579–688
Yokota O, Sasaki K, Fujisawa Y, Takahashi J, Terada S, Ishihara T, Nakashima H, Kugo A, Ata T, Ishizu H, Kuroda S (2005) Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol 12:782–790
Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P; INTERMAP Research Group (2003) Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 17:623–630
Acknowledgment
We would like to thank Ms. M. Onbe (Department of Neuropsychiatry, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences) and Ms. A. Kajitani (Department of Laboratory Medicine, Zikei Institute of Psychiatry) for collecting clinical information, and Mr. A. Sasaki for help with the creation of the manuscript. This work was supported by a grant in aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (14570957) and a research grant from the Zikei Institute of Psychiatry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yokota, O., Tsuchiya, K., Terada, S. et al. Frequency and clinicopathological characteristics of alcoholic cerebellar degeneration in Japan: a cross-sectional study of 1,509 postmortems. Acta Neuropathol 112, 43–51 (2006). https://doi.org/10.1007/s00401-006-0059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0059-7