Abstract
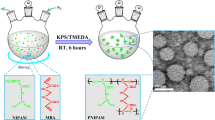
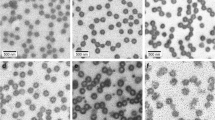
Varying size fluorescent N-isopropylacrylamide (NIPAM) nanogels were synthesized from 140 to 550 nm by precipitation copolymerization. N,N-methylenebisacrylamide (BIS) and ammonium persulfate APS were used as cross-linker and initiator, respectively. 8-Hydroxypyrene-1,3,6-trisulfonic Acid (Pyranine, Py) molecules were used for controlling the particle size and lower critical solution temperature (LCST) of nanogels. NIPAM, BIS and APS amount were kept constant and Py amount changed to tailor the particle size. Fluorescence spectroscopy, atomic force microscopy and light scattering methods show that Py caused smaller size nanogels due to the electrically charged \( S{O}_3^{-} \)side groups. Additionally, as seen from the light scattering experiment, LCST of nanogels is getting decrease considerably with increasing Py amount. Using of Py for tuning, the gel size and LCST were found very useful, easy and effective way, and it can be used for many biomedical applications.





Similar content being viewed by others
References
Pelton R (2000) Temperature-sensitive aqueous microgels. Adv Colloid Interf Sci 85(1):1–33. doi:10.1016/s0001-8686(99)00023-8
Schild HG (1992) Poly (N-isopropylacrylamide)—experiment, theory and application. Prog Polym Sci 17(2):163–249. doi:10.1016/0079-6700(92)90023-r
Chee CK, Rimmer S, Soutar I, Swanson L (2011) Time-resolved fluorescence anisotropy studies of the interaction of N-isopropyl acrylamide based polymers with sodium dodecyl sulphate. Soft Matter 7(10):4705–4714. doi:10.1039/c1sm05436h
Taylor LD, Cerankowski LD (1975) Preparation of films exhibiting a balanced temperature-dependence to permeation by aqueous-solutions—study of lower consolute behavior. Journal of Polymer Science Part a-Polymer Chemistry 13(11):2551–2570. doi:10.1002/pol.1975.170131113
Winnik FM (1990) Phase-transition of aqueous poly-(N-isopropylacrylamide) solutions—a study by nonradiative energy-transfer. Polymer 31(11):2125–2134. doi:10.1016/0032-3861(90)90085-d
Sun QH, Deng YL (2005) In situ synthesis of temperature-sensitive hollow microspheres via interfacial polymerization. J Am Chem Soc 127(23):8274–8275. doi:10.1021/ja051487k
Braun O, Selb J, Candau F (2001) Synthesis in microemulsion and characterization of stimuli-responsive polyelectrolytes and polyampholytes based on N-isopropylacrylamide. Polymer 42(21):8499–8510. doi:10.1016/s0032-3861(01)00445-1
Pich A, Richtering W (2010) Microgels by Precipitation Polymerization: Synthesis, Characterization, and Functionalization. In: Pich A, Richtering W (eds) Chemical Design of Responsive Microgels, vol 234, Advances in Polymer Science, pp. 1–37. doi:10.1007/12_2010_70
Janczewski D, Tomczak N, Han M-Y, Vancso GJ (2009) Introduction of quantum dots into PNIPAM microspheres by precipitation polymerization above LCST. Eur Polym J 45(7):1912–1917. doi:10.1016/j.eurpolymj.2009.04.005
Meng Z, Smith MH, Lyon LA (2009) Temperature-programmed synthesis of micron-sized multi-responsive microgels. Colloid Polym Sci 287(3):277–285. doi:10.1007/s00396-008-1986-8
Teng D, Hou J, Zhang X, Wang X, Wang Z, Li C (2008) Glucosamine-carrying temperature- and pH-sensitive microgels: preparation, characterization, and in vitro drug release studies. J Colloid Interface Sci 322(1):333–341. doi:10.1016/j.jcis.2009.03.014
Nagaoka N, Safranj A, Yoshida M, Omichi H, Kubota H, Katakai R (1993) Synthesis of poly(N-isopropylacrylamide) hydrogels by radiation polymerization and cross-linking. Macromolecules 26(26):7386–7388. doi:10.1021/ma00078a046
Kawaguchi H, Fujimoto K, Mizuhara Y (1992) Hydrogel Microspheres 0.3. Temperature-Dependent Adsorption of Proteins on Poly-N-Isopropylacrylamide Hydrogel Microspheres. Colloid Polym Sci 270(1):53–57. doi:10.1007/bf00656929
Zhu X, Gu X, Zhang L, Kong X-Z (2012) Preparation and characterization of nanosized P(NIPAM-MBA) hydrogel particles and adsorption of bovine serum albumin on their surface. Nanoscale Res Lett 7. doi:10.1186/1556-276x-7-519
Zhu PW, Napper DH (1996) Effects of anionic surfactant on the coil-to-globule transition of interfacial poly(N-isopropylacrylamide). Langmuir 12(25):5992–5998. doi:10.1021/la9603737
Jochum FD, Theato P (2013) Temperature- and light-responsive smart polymer materials. Chem Soc Rev 42(17):7468–7483. doi:10.1039/c2cs35191a
Yilmaz Y, Gelir A, Alveroglu E, Uysal N (2008) Testing percolation theory in the laboratory: measuring the critical exponents and fractal dimension during gelation. Phys Rev E 77(5). doi:10.1103/PhysRevE.77.051121
Alveroglu E, Gelir A, Yilmaz Y (2009) Swelling behavior of chemically ion-doped hydrogels. Macromol Symp 281:174–180. doi:10.1002/masy.200950723
Alveroglu E, Yilmaz Y (2011) Estimation of the generation and the weight fraction of dense polymer regions in heterogeneous hydrogels. Macromol Chem Phys 212(14):1451–1459. doi:10.1002/macp.201100161
Iwai K, Matsumura Y, Uchiyama S, de Silva AP (2005) Development of fluorescent microgel thermometers based on thermo responsive polymers and their modulation of sensitivity range. J Mater Chem 15(27–28):2796–2800. doi:10.1039/b502277k
Gu X, Zhu X, Kong X, Zhang L, Tan Y, Lu Y (2009) Preparation of polymer uniform microspheres via precipitation polymerization in ethanol or ethanol-water mixture as new solvent. Acta Chim Sin 67(21):2486–2494
Yilmaz Y, Uysal N, Gelir A, Guney O, Aktas DK, Gogebakan S, Oner A (2009) Elucidation of multiple-point interactions of pyranine fluoroprobe during the gelation. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy 72(2):332–338. doi:10.1016/j.saa.2008.09.012
Gelir A, Yilmaz I, Yilmaz Y (2007) In situ monitoring of the synthesis of a pyranine-substituted phthalonitrile derivative via the steady-state fluorescence technique. J Phys Chem B 111(2):478–484. doi:10.1021/jp064462w
Acknowledgments
We would like to thank Prof. Dr. Yaşar Yılmaz (Department of Physics Engineering, Istanbul Technical University) for his laboratory facilities and Prof. Dr. Fatma Z. Tepehan (Department of Physics Engineering, Istanbul Technical University) for AFM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koc, K., Alveroglu, E. Tuning the gel size and LCST of N-isopropylacrylamide nanogels by anionic fluoroprobe. Colloid Polym Sci 294, 285–290 (2016). https://doi.org/10.1007/s00396-015-3779-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3779-1