Abstract
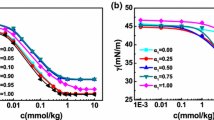
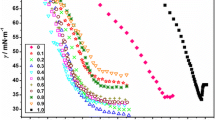
The pH dependence of an anionic surfactant, sodium N-dodecanoylsarcosinate (SLAS), has been studied by measuring interfacial tension, fluorescence, dynamic light scattering, etc., in aqueous solutions with phosphate and borate buffers. The interfacial tension (γ) of SLAS decreases remarkably with a pH decrease and is constant at pH > 7.3. The observed values for the critical micelle concentration (cmc) and the surfactant concentration at which its γ value is reduced by 20 mN/m from that of pure water (C 20) decrease with a pH decrease, while those also become constant at pH > 6.5 and >7.3, respectively. On the other hand, the interfacial excess of SLAS increases at pH < 7.3. These interfacial behaviors have been further investigated by the addition of Tl+ which replaces Na+ of SLAS. The observed γ values of LAS− with the different counter cations are in the order of H+ < Tl+ < Na+. In order to reveal aggregation properties of SLAS, the aggregation number (N agg), the micropolarity, the hydrodynamic radius (R h) of micelle, and the fluorescence anisotropy of Rhodamine B (r) have been evaluated at various pHs. The N agg value shows a decreasing tendency with a pH increase. The I 1/I 3 ratio and the R h values do not strongly depend on pH. The r value decreases until pH 7 and remains constant at pH > 7.0. These interfacial and micelle properties have been discussed in detail considering the electrostatic interaction and the molecular structures of the hydrophilic headgroup.









Similar content being viewed by others
References
Yumioka R, Koyama M (2000) Novel emollients derived from amino acids. Proceedings of XXI IFSCC Congress, Poster #82 Sep, Berlin
Yamashita Y, Kunieda H, Oshimura E, Sakamoto K (2003) Phase behavior of N-acylamino acid surfactant and N-acylamino acid oil in water. Langmuir 19:4070–4078
Gad EAM, El-Sukkary MMA, Ismail DA (1997) Surface and thermodynamic parameters of sodium N-acyl sarcosinate surfactant solutions. J Am Oil Chem Soc 74:43–47
Miyagishi S, Higashide M, Asakawa T, Nishida M (1991) Surface tensions and critical micelle concentrations in mixed solutions of potassium perfluorononanoylalaninate and potassium acylalaninates. Langmuir 7:51–55
Rosen MJ, Cohen AW, Dahanayake M, Hua XY (1982) Relationship of structure to properties in surfactants. 10. Surface and thermodynamic properties of 2-dodecyloxypoly (ethenoxyethanol)s, C12H25(OC2H4)xOH, in aqueous solution. J Phys Chem 86:541–545
Dahanayake M, Cohen AW, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100:5951–5952
Jobe DJ, Verrall RE (1990) Polarized fluorescence emission measurements in mixtures of 2-butoxyethanol, cetyltrimethylammonium bromide, and water. Langmuir 6:1750–1757
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York, pp 353–382
Rosen MJ (2004) Surfactants and interfacial phenomenon, 3rd edn. John Wiley & Sons, Hoboken, pp. 150 and 34–177
Tomoaia-Cotisel M, Zsako J, Mocanu A, Lupea M, Chifu E (1987) Insoluble mixed monolayers. III. The ionization characteristics of some fatty acids at the air/water interface. J Colloid Interface Sci 117:464–476
Cren EC, Morelli AC, Sanches T, Rodrigues CE, Meirelles AJA (2010) Adsorption isotherms for removal of linoleic acid from ethanolic solutions using the strong anion exchange resin amberlyst A26 OH. J Chem Eng Data 55:2563–2566
Sharma KS, Rodgers C, Palepu RM, Rakshit AK (2003) Studies of mixed surfactant solutions of cationic dimeric (gemini) surfactant with nonionic surfactant C12E6 in aqueous medium. J Colloid Interface Sci 268:482–488
Moulik SP, Haque ME, Jana PK, Das AR (1996) Micellar properties of cationic surfactants in pure and mixed states. J Phys Chem 100:701–708
Stevens B, Sharpe RR, Bingham WSW (1967) pH dependence of Rhodamine B semiquinone dismutation rate in aqueous alcoholic solution. Photochem and Photobio 6:83–89
Bordes R, Tropsch J, Holmberg K (2010) Role of an amide bond for self-assembly of surfactants. Langmuir 26:3077–3083
Tajima K, Imai Y, Nakamura A, Tsubone K, Mimura K, Nakatsuji Y, Ikeda I (2001) Specific surface activity of Gemini surfactants: mixing effects of alanine-type and sulfate-type surfactants. J Oleo Sci 50:453–462
Mukherjee S, Dan A, Bhattacharya SC, Panda AK, Moulik SP (2011) Physicochemistry of interaction between the cationic polymer poly(diallyldimethylammonium chloride) and the anionic surfactants sodium dodecyl sulfate, sodium dodecylbenzenesulfonate, and sodium N-dodecanoylsarcosinate in water and isopropyl alcohol-water media. Langmuir 27:5222–5233
Tsubone K, Rosen MJ (2001) Structural effect on surface activities of anionic surfactants having N-acyl-N-methylamide and carboxylate groups. J Colloid Interface Sci 244:394–398
Takahashi H, Nakayama Y, Hori H, Kihara K, Okabayashi H, Okuyama M (1976) The proton magnetic resonance spectra and molecular conformations of sodium N-acyl sarcosinates in aqueous solution. J Colloid Interface Sci 54:102–107
Ambuehl M, Bangerter F, Luisi PL, Skrabal P, Watzke HJ (1993) Configurational changes accompanying vesiculation of mixed single-chain amphiphiles. Langmuir 9:36–38
Acknowledgments
We are grateful to Prof. H. Tsukube (Graduate School of Science, Osaka City University, Japan) for his helpful assistance in the steady-state fluorescence and dynamic light-scattering measurements, to Prof. T. Nagasaki (Graduate School of Engineering, Osaka City University, Japan) for his helpful assistance in the fluorescence anisotropy measurements, and also to Prof. Y. Morimoto (Graduate School of Science, Osaka City University, Japan) for his helpful comments on the discussion of resonate structures, (Z)-LAS− and (E)-LAS−. PS is grateful to the Japan Society for the Promotion of Science (JSPS) for postdoctoral fellowship award.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 97 kb)
Rights and permissions
About this article
Cite this article
Kaneko, A., Sehgal, P. & Doe, H. Interfacial and aggregation properties of aqueous sodium N-dodecanoylsarcosinate solutions at different pH. Colloid Polym Sci 290, 323–330 (2012). https://doi.org/10.1007/s00396-011-2551-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-011-2551-4