Abstract
Immune checkpoint inhibitor (ICI) therapy represents a ground-breaking paradigm in cancer treatment, harnessing the immune system to combat malignancies by targeting checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). The use of ICI therapy generates distinctive immune-related adverse events (irAEs) including cardiovascular toxicity, necessitating targeted research efforts. This comprehensive review explores preclinical models dedicated to ICI-mediated cardiovascular complications including myocarditis. Tailored preclinical models of ICI-mediated myocardial toxicities highlight the key role of CD8+ T cells, emphasizing the profound impact of immune checkpoints on maintaining cardiac integrity. Cytokines and macrophages were identified as possible driving factors in disease progression, and at the same time, initial data on possible cardiac antigens responsible are emerging. The implications of contributing factors including thoracic radiation, autoimmune disorder, and the presence of cancer itself are increasingly understood. Besides myocarditis, mouse models unveiled an accelerated progression of atherosclerosis, adding another layer for a thorough understanding of the diverse processes involving cardiovascular immune checkpoint signalling. This review aims to discuss current preclinical models of ICI cardiotoxicity and their potential for improving enhanced risk assessment and diagnostics, offering potential targets for innovative cardioprotective strategies. Lessons from ICI therapy can drive novel approaches in cardiovascular research, extending insights to areas such as myocardial infarction and heart failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
In recent years, immune checkpoint inhibitor (ICI) therapy has revolutionized cancer treatment by leveraging the body’s immune system to fight malignancies. In contrast to conventional chemotherapeutics, which in most cases target proliferating properties or specific aberrations of cancer cells, ICIs target immune checkpoints at various stages to induce a specific anti-tumor immune response.
The initiation of an adaptive immune response depends on the crucial step of T cell activation in response to antigen presentation. This activation is triggered by the recognition of antigens by the T cell receptor (TCR). To facilitate this process, multiple costimulatory signals are required. Immune checkpoints play a vital role in counterbalancing T cell activation, preventing an overly exaggerated immune response, and ensuring self-tolerance. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is an immune checkpoint that dampens T cell activation by vying with CD28 for binding to B7 proteins on antigen-presenting cells (APCs). This regulatory process prevents overactive immune responses, preserving immune homeostasis and self-tolerance [75]. Similarly, during the process of T cell activation, programmed cell death protein 1 (PD-1) is expressed and functions to counterbalance positive signals from the TCR and CD28. This is accomplished through interactions with its ligands, programmed cell death 1 ligand 1 and programmed cell death 1 ligand 2 (PD-L1 and PD-L2) [78].
Targeting immune checkpoints has become a promising treatment option for different malignancies. Receiving the first regulatory endorsement in 2011 for the treatment of unresectable or metastatic melanoma, ICIs are now in use for different tumor entities including lung cancer, hepatocellular carcinoma, ovarian cancer, and renal cell carcinoma amongst others. The administration of these medications has resulted in enduring treatment responses and, in some cases, complete remission in patients with advanced-stage cancer [4, 70].
One downside to these breakthroughs is the onset of a novel range of immune-related adverse events (irAEs), which differ markedly from the traditional toxicities associated with chemotherapy. These irAEs include colitis, pneumonitis, and myocarditis which stands out with the highest fatality rate [53, 59, 84, 85]. Initially considered infrequent and rare, cardiotoxic side effects are now recognized to affect a substantial number of patients undergoing treatment with ICIs, with a 1-year absolute risk of cardiac events reaching up to 9.7% [16]. Nevertheless, variations exist in the incidence of cardiotoxicity-related incidents among ICIs. Typically, ICIs aimed at PD-1 and the combined inhibition of PD-1 and CTLA4 show elevated rates of cardiotoxicity, with the incidence in dual checkpoint inhibition being 87% higher than in monotherapy. Forty percent of patients who developed ICI-induced cardiotoxicity had pre-existing cardiovascular risk factors [43, 76]. The negative impacts mentioned present a notable hurdle to the continued utilization of ICIs. As a result, various preclinical models have been developed to better understand ICI-induced cardiotoxicity, aiming to clarify potential mechanisms for addressing these adverse effects. Three main facets of ICIs within the cardiovascular system are addressed by preclinical models (Fig. 1):
-
1.
The role of immune checkpoint signaling for basic cardiovascular function. Broad cardiovascular effects affecting all parts of the cardiovascular system with a diverse phenotype indicate a relevant role of immune checkpoints for cardiovascular function at baseline. The putative involvement of immune checkpoints encompasses preventing overactive immune responses, preserving cardiac immune equilibrium, and exerting distinct effects on myocardial function, metabolic signaling, and metabolic pathways [2, 26, 32, 82].
-
2.
Predictors and responsible pathways in cardiovascular involvement during ICI therapy. ICI therapy was shown to induce a wide range of cardiovascular complications, including myocarditis, left ventricular (LV) dysfunction, arrhythmia, and progression of coronary artery disease [5, 37, 48, 60]. A general involvement of key cardiovascular pathways can be demonstrated by various preclinical models, indicating a complex biological phenotype of these complications. Understanding additional risk factors that determine phenotype and severity will be challenging for future research.
-
3.
Cardiovascular epitopes in ICI-related cardiovascular autoimmune reactions. Identifying autoantigens that drive ICI-related autoimmune reactions against the cardiovascular system is the focus of current research. Recent data suggest cardiac α-myosin as a potential autoantigen, while another work found evidence for a potential shared epitope between cancer and cardiovascular tissue [8, 43, 86]. Identifying responsible antigens comes with great potential for the development of a targeted cardioprotective approach.
Scientific databases were screened to identify preclinical models of ICI-related cardiotoxicity. Publications until March 2024 are incorporated herein. In total, this review focuses on 15 models for ICI-induced myocardial toxicity described in 19 publications and 10 models for ICI-induced vascular toxicity.
Myocardial toxicity
Occurrence of myocarditis following ICI therapy was initially recorded as case reports and small case series [43]. However, it became apparent that the incidence exceeded initial estimations, and cardiotoxicity other than myocarditis may manifest in a significant number of cases, presenting as LV dysfunction or asymptomatic troponin elevation [13, 52, 57]. Considering these clinical observations, preclinical models are essential to understand the diverse pathophysiology implicated in these heterogeneous presentations of ICI-mediated cardiotoxicity (Fig. 2). Preclinical models for myocardial inflammation designed to study ICI-induced cardiotoxicity will be elaborated in the sections that follow.
Mediators of ICI-induced cardiotoxicity in preclinical models. GM-CSF granulocyte–macrophage colony-stimulating factor, HSP heat shock protein, ICAM intercellular adhesion molecule 1, IFNγ interferon γ, iNOS inducible nitric oxide synthase, MANF mesencephalic astrocyte-derived neurotrophic factor, PD-L1 programmed cell death 1, Tregs regulatory T cells, TNF-α tumor necrosis factor α, VCAM vascular cell adhesion molecule. Created with BioRender.com
The role of PD-1/PD-L1 in ICI-mediated cardiotoxicity
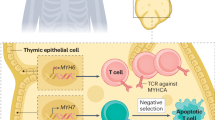
MRL (Murphy Roths large)/faslpr/lpr mice serve as a murine model for a systemic autoimmune syndrome analogous to systemic lupus erythematosus [96]. This model susceptible to autoimmune diseases was used and, by backcrossing with a C57BL/6 background, MRL-Pdcd1−/− mice were established that developed fatal myocarditis along with infiltration of CD4+ and CD8+ T cells and myeloid cells. The predominant activity of type 1 T helper (Th) cells and their associated cytokines including interferon γ (IFNγ) was attributed to the observed myocarditis in MRL-Pdcd1−/− mice. Interestingly, while hepatitis, pneumonitis, gastritis, and sialadenitis were evident, myocarditis was absent in MRL wild-type mice. This underscores the crucial role of PD-1 in regulating the immune response within cardiac tissue. Notably, PD-L1 expression was heightened in cardiomyocytes within inflamed hearts, suggesting a potential mechanism involving the suppression of autoreactive T cells expressing PD-1. The authors identified strong auto-antibody production against cardiac myosin in MRL-Pdcd1−/− mice. In contrast, MRL mice showed weak antibody production against cardiac myosin prompting that Pdcd1 deficiency might open the door for the development of myocarditis inclination in MRL mice [86]. Accordingly, PD-L1−/−;MRL−Faslpr mice died earlier than their non-PD-L1-deficient controls due to myocarditis-induced heart failure with myocyte destruction orchestrated by macrophages and T cells. The phenotype was not dependent on the Faslpr mutation, suggesting that the observed myocarditis and pneumonitis were determinant on the MRL background. The transfer of hematopoietic bone marrow cells was sufficient to induce myocarditis and pneumonitis in wild-type mice. Since cardiac-specific autoantibodies against cardiac troponin I and myosin were shown to increase only after the onset of overt disease, autoantibodies were not considered as key drivers of disease manifestation [50]. Treatment with anti-PD-1 antibodies in A/J mice induced multiorgan inflammation with affection of the heart in 19%. Cardiac T cells showed an activated phenotype, and activation of T cells specific for cardiac myosin was observed. PD-1+ cardiac myosin-specific T cells were detectable in naïve mice hinting that ICIs might reactivate those autoreactive T cells leading to ICI-related cardiotoxicity. The administration of interleukin-12 increased the incidence of PD-1 inhibitor-induced myocarditis which was attributed to the enhanced differentiation of pathogenic effector CD8+ T cells [32, 91]. Another group identified antibodies against cardiac troponin I as the possible driver for the onset of dilative cardiomyopathy in BALB/c-Pdcd1−/− mice. The phenotype was not present in BALB/c-Rag-2−/− mice underlining that the impaired myocardial function in BALB/c-Pdcd1−/−mice is linked to the functions of T and/or B lymphocytes as Rag-2−/− mice are incapable to produce mature B or T lymphocytes [67, 69, 79]. In an experimental autoimmune-related myocarditis mouse model induced by immunization with murine cardiac troponin I peptide, the immunoproteasome was found to regulate CD4+ T cell activation and differentiation towards amplified Th17 and Th1 expansion, while also stimulating proinflammatory cytokines. Deletion of either Low-molecular-weight protein 2/7 as parts of the immunoproteasome or administration of an immunoproteasome inhibitor reduced inflammation and improved cardiac function. Similar inflammatory pathways observed in this murine model have been detected in clinical cases of ICI-related myocarditis, suggesting troponin I as a putative autoantigen [9].
To delineate the specific roles of CD4+ and CD8+ T cells in the pathogenesis of ICI-related cardiotoxicity, two distinct mouse models of T cell-dependent myocarditis were employed. While the cMy-mOva model of myocarditis relies on CD8+ effector T cells, the experimental autoimmune myocarditis (EAM) model in BALB/c mice utilized in research is triggered by CD4+ T cells. In both models, the authors effectively illustrated that PD-1 exerts a substantial regulatory role in controlling T cell-mediated inflammation within cardiac tissue [2, 32, 82]. The potential impact of PD-1 on cardiac endothelium was evidenced by the exacerbation of phenotype progression in the acute lymphocytic myocarditis model upon PD-L1 depletion [33].
Further evidence that PD-1/PD-L1 interaction maintains cardiac integrity comes from the mouse model of chronic Chagas disease. Chagas disease is conveyed through the transmission of Trypanosoma cruzi (T. cruzi), potentially resulting in Chagas cardiomyopathy following the prolonged persistence of the parasitic organisms. In T. cruzi-induced acute myocarditis in mice, the introduction of blocking antibodies targeting PD-1 and PD-L1 resulted in an elevated presence of inflammatory infiltrates in the myocardium, accompanied by heightened levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and IFNγ. Remarkably, uninfected C57BL/c-Pdcd1−/− mice exhibited no indications of myocarditis [35]. Although the combined administration of PD-1/PD-L1 blockade and immunization with irradiated T. cruzi led to a decrease in blood parasitemia, it did not affect cytokine production in the heart tissue, contrary to earlier observations [7, 35].
In a different approach, the effects of PD-1 blockade in C57BL/6 mice that underwent targeted radiation of the heart were evaluated. Animals receiving both radiation and PD-1 blockade showed increased mortality, decreased cardiac output along with increased fibrosis and myocardial inflammation. The authors attribute the cardiac toxicities to cytotoxic CD8+ T cells as depletion reversed mortality from radiation and PD-1 blockage [17]. Similarly, the application of full thoracic X-ray irradiation to C57BL/6 mice that received anti-PD-1 antibodies increased T cell influx into the heart and lung in comparison to animals that underwent sole irradiation. The combination of irradiation and immunotherapy reduced the 21-day survival rate from 70% (irradiation) to 36% (anti-PD-1 plus irradiation) [64]. This perspective holds significant importance as it clarifies the complexities of combinatorial cancer treatment, offering valuable insights into its practical implementation [61].
Recently, preprint data suggested an increase in CXCL9/10+ CCR2+ macrophages and emergence of CXCR3hi CD8+ T cells in MRL-Pdcd1−/− mice. Bioinformatic analyses indicated an anticipated interaction between these macrophages and T cells. Depletion of macrophages via liposomal clodronate administration resulted in decreased clonal expansion of CD8+ T cells in murine hearts and improved overall survival. In MRL mice treated with anti-PD-1 and anti-CTLA-4 antibodies, CXCR3 blockade attenuated immune cell infiltration in the heart, leading to increased survival, particularly by targeting CXCR3hi CD8+ T cells. CXCL9/10+ macrophages and CXCR3hi CD8+ T cells were also found in biopsies from human hearts affected by ICI-myocarditis, suggesting their interaction as a potential novel target for pharmaceutical treatment of ICI-myocarditis [40].
C57Bl/6J mice treated with clinically used doses of pembrolizumab demonstrated early activation of Th17-type cells and mild impairment in systolic function after 1 week. Subsequent intracardial infiltration of T helper cells at 2 weeks exacerbated systolic dysfunction, accompanied by macrophage infiltration, and histologically defined cardiac abnormalities at five weeks. Pembrolizumab administration induced coronary endothelial dysfunction with increased levels of intercellular adhesion molecule 1 (ICAM-1), inducible nitric oxide synthase (iNOS) and significant upregulation of endothelial activation markers like E-selectin. High concentrations of atorvastatin prevented ICI-induced endothelial toxicity in human endothelial cells in vitro and mitigated progressive cardiac dysfunction, cardiac injury, and acute Th17 cytokine storm in the murine model, suggesting its potential as a prophylactic agent against ICI-induced cardiotoxicity [19, 20].
In conclusion, these findings underscore the critical role of PD-1 signaling in maintaining cardiac integrity and regulating immune responses in various models of myocarditis and cardiotoxicity, while inflammatory processes, the interaction of immune cells (e.g. T cells) and immunomodulatory substrates (e.g. TNF-α and IFNγ) present as the driving factors for the induced phenotype.
The role of CTLA-4 and LAG-3 in ICI-mediated cardiotoxicity
Like PD-1 and PD-L1, CTLA-4 is a key player in preserving peripheral tolerance to the heart. Mice lacking Ctla4 show a phenotype similar to that of Pdcd1-deficient mice, but more severe. They display massive accumulation of T cell blasts in liver, heart, lung, and pancreatic tissue dying by 3–4 weeks of age [83, 87]. Differing from the Pdcd1 knockout, the phenotype was present in Ctla4-deficient mice on both C57BL/6 and BALB/C backgrounds, together with markedly increased levels of IFNγ, interleukin-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) as an indicator of T cell activation [83]. Studies compared the impact of abrogating CTLA-4 expression in adult mice compared to congenitally Ctla4-depleted mice. Whereas multiorgan lymphocyte infiltration was present in both models, the development of myocarditis was unique to mice born with Ctla4 deficiency. This indicates that autoimmune reactions in mice lacking CTLA-4 display unique organ preferences depending on the specific types of CTLA-4 depletion [45]. In the model of EAM, anti-CTLA-4 treatment of BALB/c mice significantly exacerbated the disease with intense infiltration of lymphocytes into the heart, accompanied by a notable rise in the percentage of CD3+ T cells. The authors hypothesized that blocking CTLA-4-B7 interaction enhances Th17-mediated autoimmune responses. This was underlined as the neutralization of IL-17 significantly suppressed the development of the EAM [94]. Another research group corroborated these findings by demonstrating that cardiac function during anti-PD-1 therapy was preserved during specific inhibition of IL-17A. Although minimal myocardial inflammatory changes accompanied by elevated nitrosative stress were evident, an upregulation of thymic cytokine expression highlighted T cell activation within the thymus as a potential mediator of ICI-induced myocarditis. BALB/c mice, characterized by a Th2-dominant immune response, did not display compromised cardiac function. This observation led the authors to propose that discrepancies in systemic immune responses to PD-1 inhibition may predispose individuals to cardiotoxicity development [27].
CD8+ T cells were pinpointed as the catalyst for myocarditis in Pdcd1−/− Ctla4+/− mice. The survival of these mice markedly enhanced following the depletion of CD8+ cells, with the removal of CD4+ cells showing no substantial impact. Akin to previous reported results, the cardiac-specific protein α-myosin was identified as the origin of the cognate antigen for three major histocompatibility complex class I-restricted TCRs derived from mice undergoing fulminant myocarditis [86]. Taking a translational approach, T cells from the peripheral blood of three patients experiencing ICI-related myocarditis exhibited a robust expansion induced by α-myosin peptides making α-myosin a putative autoantigen in ICI-myocarditis [8].
To further elucidate the role of CTLA-4 on forkhead-Box-Protein P3 (Foxp3+) regulatory T cells (Tregs), the influence of specifically deleting the Ctla4 gene in Foxp3+ Tregs in mice on BALB/c background was evaluated. Whereas Ctla4 knockout mice became moribund at ∼ 20 days of age, Ctla4 deficiency in Tregs alone showed a less profound phenotype with moribundity after 7 weeks of age. Mice lacking Ctla4 in Tregs exhibited dense infiltration of mononuclear cells into the myocardium, resulting in the destruction of myocytes and possibly leading to fulminant myocarditis as cause of death. Transferring the splenocytes and purified CD4+ T cells into T cell-deficient BALB/c athymic nude mice led to myocarditis, indicating that this condition is T cell-mediated. In both the spleen and lymph nodes, an increased frequency of interleukin-2-, interleukin-4-, and IFNγ-producing Foxp3+ CD4+ cells were observed. Supporting previous findings, the authors described a rise of IL-17-secreting (Th17) cells in Ctla4-deficient mice which was not present in Ctla4 deficiency in Tregs alone, possibly linking Th17 to accelerated progression to fulminant disease [90, 94]. In a murine transgenic model of CD8+ T cell-mediated myocarditis in Ctla4−/− mice, CD8+ T cells dependent on interleukin-12 were identified as a key factor driving inflammation underlining that it is hard to attribute cardiotoxicity of ICIs to one sole interleukin [49].
A transgenic mouse model carrying loss-of-function alleles of Ctla4 and Pdcd1 was engineered to explore the effects of T cells and the resulting phenotype in rodents. As documented before, the homozygous loss of Ctla4 resulted in extensive and lethal lymphoproliferation [83, 87]. In comparison to Ctla4+/+ Pdcd1−/− mice, Ctla4+/− Pdcd1−/− mice showed a more pronounced phenotype with lymphocytic infiltration (mainly CD3+ CD8+ T cells, in a lesser extent CD4+ T cells, low amount of Foxp3+ Tregs) into the myocardial tissue and myocyte destruction. Whereas no statistically significant differences in echocardiographic parameters were observed, electrocardiographic recordings revealed arrhythmias including sinus node dysfunction, sinus arrest, atrioventricular conduction block, and severe bradycardia in Ctla4+/− Pdcd1−/− mice. The administration of abatacept (recombinant CTLA-4 Ig) reduced mortality and normalized myocardial immune infiltrates in Ctla4+/− Pdcd1−/− mice [88].
Sex-related differences in cardiotoxicity are observed in clinical and preclinical data, with females at higher risk [1]. Female C57BL/6J tumor-bearing mice showed more CD8+ T cell infiltration and impaired cardiac function after anti-PD-1 and anti-CTLA-4 treatment compared to males. This may be due to lower expression of cardioprotective mesencephalic astrocyte-derived neurotrophic factor and heat shock protein 5 in female mice [28, 29, 97]. Administration of these proteins as recombinant factors attenuated ICI-induced myocarditis. ICI treatment reduced 17-β-estradiol levels in both male and female mice, and activation of estrogen receptor β signaling improved cardiac function and reduced myocardial infiltration by CD8+ T cells [98].
Unlike all murine models of ICI-mediated toxicities, a monkey model was established by administering nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) to Chinese-origin cynomolgus monkeys. In addition to various organ toxicities, myocarditis was also detected. Transcriptomic analysis revealed heightened migration and activation of T cells, along with increased phagocytosis and antigen presentation within the cardiac tissue. The infiltration of mononuclear cells in the myocardium was primarily comprised of T cells, with fewer macrophages and occasional B cells present. This infiltration was associated with minimal cardiomyocyte degeneration, along with elevated levels of cardiac troponin I and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) [42].
With the evolution of combinatorial treatment involving various ICIs, there is a pressing necessity to understand the effects of deficiencies in various immune checkpoints on individuals. Initially aiming to introduce a rodent model that is defective in random somatic hypermutation and class switch recombination, a mouse model of spontaneous autoimmune disease was discovered. The autoimmune phenotype in those Aicda−/− mice was not due to the deficiency in activation-induced cytidine deaminase (Aicda) but rather due to spontaneous loss-of-function mutation in the neighboring gene lymphocyte-activation gene 3 (Lag3). Aicda−/− Pdcd1−/− mice on a BALB/c background died before 10 weeks of age exhibiting dilatation of the heart along with massive lymphocytic infiltration into the atrium and ventricle of the heart. Tregs in BALB/c-Pdcd1−/− Lag3aida/aida mice had substantial suppressive function, contradicting that myocarditis in this model is mainly orchestrated by the failure of regulatory cells. As Lag3 deficiency did not induce autoimmunity in non-autoimmune-prone mouse models, the synergistic actions of LAG-3 and PD-1 are crucial in mediating the autoimmunity [68]. Corroborating this, it was demonstrated that Lag3−/− Pdcd1−/− mice exhibited an early-onset lethal autoimmune condition, manifesting with endocarditis, myocarditis, and pancreatitis [92].
Early cardiac dysfunction as a manifestation of ICI cardiotoxicity
Our research group was part of the development of a murine melanoma model showing response to anti-PD-1 therapy by transplantation of cutaneous melanoma cells into immunocompetent wild-type C57BL/6N mice. Anti-PD-1 therapy induced a loss of tumor volume along with strong intratumoral recruitment of CD4+ and CD8+ T cells. Upon administering anti-PD-1 therapy, there was an upregulation of PD-L1 on cardiac endothelial cells. Moreover, notable alterations impacting cellular energy production and equilibrium were detected. Substrates for beta-oxidation significantly increased, while levels of carnitine/acylcarnitine carrier protein, acyl-CoA dehydrogenase, and acyl-CoA synthetase decreased concurrently. Elevated cardiolipin levels were globally observed, suggesting mitochondrial dysfunction after ICI administration. The identified alterations resulted in baseline LV dysfunction and failure to respond to inotropic stress. The removal of CD8+ T cells induced the loss of anti-tumor efficacy in anti-PD-1 therapy. In contrast, blocking TNFα reduced cardiotoxicity, as evidenced by maintained LV function alongside preserved anti-tumor response. In mice subjected to anti-PD-1 therapy, the inhibition of TNFα did not impact the augmented recruitment of infiltrating lymphocytes. Still, it resulted in an intensified expression of the immune-inhibitory LAG3, T cell immunoglobulin, and mucin-domain containing-3 (TIM3), indicative of markers associated with lymphocyte exhaustion [56].
Macrophages are abundant within cardiac tissue, and recent research has unveiled their pivotal role in maintaining cardiovascular integrity. In addition to modulating tissue metabolism, they serve as central regulators of immune response within the cardiac microenvironment [24, 46, 66]. Their influence on disease pathology is evident in murine models of chronic heart failure, where the proliferation of proinflammatory macrophages contributes to sustained pathological remodeling [77]. It was demonstrated that the administration of PD-1 inhibitors leads to cardiac function impairment portrayed by LV impairment assessed 28 days after administration of ICIs, accompanied by evident macrophage polarization towards a proinflammatory M1 phenotype both in vivo and in vitro. In C57/Bl6 mice treated with anti-mouse PD-1 inhibitor, proinflammatory macrophage expansion of inducible Nitric Oxygen Synthase (iNOS+) M1 macrophages were observed along with heightened interleukin-1β, interleukin-6, and TNF-α. The restoration of cardiac function, impaired by a PD-1 inhibitor, was achieved by inhibiting MicroRNA-34a (miR-34a), a regulator of macrophage polarization that induces inflammation in cultured macrophages [93].
The previous studies support the idea that treatment involving ICIs induces subclinical alterations in cardiac function, as evidenced by LV dysfunction observed in echocardiography [26]. It is crucial to clarify the factors contributing to the progression of subclinical changes to overt cardiovascular disease in certain instances.
Dual-hit hypothesis
Models of myocarditis triggered by ICI therapy commonly embrace a dual-hit hypothesis. These models encompass various properties, including susceptibility to immunological phenomena (MRL and BALB/c mice), myocarditis models (cMy-mOva mice and experimental myocarditis models), infectious disease models (Chagas disease model), radiation-induced models (irradiation in mice), and cancer models (melanoma model) (Fig. 3). Moreover, the dual blockade of multiple immune checkpoints has been shown to enhance the observed effects in these models. Hence, latent alterations at both the molecular and immunological levels induced by ICIs might subsequently evolve into overt cardiovascular toxicity when confronted with an additional predisposing condition or risk factor. Within this framework, the heart could potentially become susceptible to the proinflammatory effects of ICI therapy under any form of cardiac stress. This aligns with the notion of hidden cardiotoxicity, where the toxic effects on the heart from a drug are only apparent in a diseased heart, underscoring the importance of identifying predisposing factors. The dual-hit hypothesis extends the concept of hidden cardiotoxicity by including extracardiac proinflammatory effects as systemic stressors that promote progression from subclinical changes to manifest cardiovascular toxicity [22].
Preclinical models of ICI-mediated cardiotoxicity with different manifestations of cardiac inflammation. Aicda activation-induced cytidine deaminase, Ctla4 cytotoxic T-lymphocyte-associated protein 4 gene, ECG electrocardiography, HSP heat shock protein, ICAM-1 intercellular adhesion molecule 1, ICI immune checkpoint inhibitor, IL-1β interleukin-1β, IL6 Interleukin-6, MANF mesencephalic astrocyte-derived neurotrophic factor, miR-34a MicroRNA-34a, MRL Murphy Roths large, NOS nitric oxygen synthase, NT-proBNP N-terminal pro-B-type natriuretic peptide, Pdcd1 programmed cell death protein 1 gene, TNF-α tumor necrosis factor α, Tregs regulatory T cells. Created with BioRender.com
Atherosclerosis in models for ICI cardiotoxicity
For more than 2 decades, it has been established that atherosclerosis is not the sole accumulation of lipids in arteries; rather, it is an intricate pathophysiological process primarily driven by inflammatory mechanisms [73]. These processes are orchestrated by both the innate and the adaptive immune system and are heavily dependent on the activity of monocytes, macrophages, Th cells, and the release of cytokines [80]. Understanding and eventually throttling the immunologically mediated progression from dysfunctional endothelial lesions to clinically manifest atherosclerosis is a major goal. A meta-analysis highlighted that the development of atherosclerotic-related cardiovascular events in patients under ICI exposure is more likely than in their controls with an odds ratio of 1.51 for myocardial infarction. Therefore, it is crucial to comprehend the pathways of ICI-mediated atherosclerotic effects in preclinical models [14, 15]. To attain this objective, extensively utilized methods include genetic knockout mouse models and pharmacological modulation through ICIs. Studies so far mainly focused on the target proteins CTLA-4, PD-1/PD-L1, and LAG3.
In Ldlr−/− mice (low-density lipoprotein receptor knockout mouse model) that are commonly used as a model for hypercholesterinemia, short-term anti-CTLA-4/anti-PD-1 treatment did not influence the atherosclerotic plaque area per se. Nonetheless, ICI treatment led to more advanced atherosclerosis plaques with more pathological intima thickening, fibrous cap atheromas, and an increase in plaque necrotic core area. This went along with more CD8+ T cell infiltration possibly provoking an increase in necrotic core size of atherosclerotic plaques through macrophage death. The elevated presence of adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) and ICAM-1 on the arterial endothelium might have facilitated the infiltration of T cells into the arterial wall of mice treated with ICIs [71]. The same research team also investigated how the antibody-mediated inhibition of CTLA-4 aggravates atherosclerotic plaque inflammation in Ldlr−/− mice. CTLA-4 treatment induced an activated T cell profile and endothelial activation. Atherosclerotic plaque area was found to be twice as large with a more advanced morphological phenotype and an increased T cell/macrophage ratio [72].
The overexpression of CTLA-4 in apolipoprotein E-deficient mice ((CTLA-4-Tg)/Apoe(-/-)) resulted in diminished formation of atherosclerotic lesions and reduced intraplaque accumulation of macrophages and CD4+ T cells in the aortic root. This went along with a decreased expression of the costimulatory molecules CD80 and CD86 acting as ligands for CTLA-4 as well as the costimulatory molecule CD28 on dendritic cells [54]. Blockage of this costimulatory CD-28-CD80/86-T cell activation through abatacept-treatment decelerated atherosclerosis in ApoE3*Leiden mice acting as a common preclinical model of accelerated atherosclerosis [21]. Likewise, in a model of hyperhomocysteinemia (HHcy)-accelerated plaques in apolipoprotein E-deficient (apoE–/–) mice abatacept-treatment reversed plaque development with reduced T cell-dependent macrophage recruitment and IFN-γ/Interleukin-2 secretion [51]. Similar to those discoveries, it was demonstrated that Pdl1−/− Ldlr−/− mice developed larger atherosclerotic lesions compared to Ldlr−/− mice. More abundant CD4+ and CD8+ T cells and macrophages were part of those lesions [10]. Similarly, after 10 weeks of high-cholesterol diet feeding, the intima of Pdl1/2–/–LdlrR–/– mice showed a threefold increase in CD4+ T cells as well as a marked increase in CD8+ T cell infiltration in the intima. This coincided with a general rise in the atherosclerotic burden observed in the aortic sinus and arch of these animals.
The PD-1/PD-L pathway is believed to limit atherosclerosis by downregulating proatherogenic T cell response and limiting APC-dependent T cell activation [31]. Echoing this hypothesis and underscoring the distinctive impact on specific T cell populations, the stimulation of the PD-1 pathway in Ldlr−/− mice led to a decrease in atherogenic IFNγ-producing splenic CD4+ T cells, whereas atheroprotective IL-10-producing CD4+ T cells were elevated. Overall, the diminished immune activation following the administration of agonistic PD-1 antibodies reduced atherosclerosis development [34]. In reperfused acute myocardial infarction (repAMI), anti-PD-1 therapy fosters the infiltration of CD8+ T cells into the myocardium, without influencing the sizes of the infarct. There was no significant alteration observed in the count of B cells and CD4+ T cells. Nonetheless, these findings underline the immediate effects of ICIs on cardiovascular integrity [58].
The effects of ICI therapy seem to be diverse and involve different immunological pathways as it was outlined that interrupting PD-1 signaling boosts not only the widespread activation of proatherogenic IFN- γ releasing Th1 cells but also of atheroprotective Foxp3+ Tregs. However, the authors showed in their studies using Ldlr−/−Pdcd1−/− mice that the proatherogenic effects prevail as shown by enhanced dyslipidemia, vascular inflammation, and atherosclerosis [11].
The immune checkpoint receptor LAG3 is the most recent target for immune checkpoint therapy, with recent FDA approval for antibody treatment [63]. LAG3 is expressed on different subsets of leucocytes and mainly acts through interaction with its ligands MHC Class II, Galectin-3, and LSECtin. Although not yet fully understood LAG3 regulates T cell function and expansion [6, 41, 47]. Murine hypercholesterolemic models of atherosclerosis were utilized to investigate the role of LAG3 in the disease process. While not promoting atherosclerotic plaque size, both Lag3-deficient and anti-LAG3 treated mice showed enhanced density of T cells in plaques compared to controls. Further, levels of IFN-γ-producing Th1 cells, effector/memory T cells, and regulatory T cells were increased. In particular, the combinatorial blockage of PD-1 and LAG3 increased those effects compared to the mono-blockage of LAG3 [62].
The existing preclinical evidence emphasizes the potential hazard posed by immune checkpoint inhibitor-induced exaggerated atherosclerosis. The available evidence indicates that the proatherosclerotic effects primarily stem from heightened T cell-modulated inflammation within atherosclerotic plaques after ICI administration (Table 1). A more profound comprehension of these mechanisms holds the promise of mitigating the impact on atherosclerotic progression following ICI treatment and concurrently advancing our insights into the broader therapeutic landscape for atherosclerotic diseases. Thus far, preclinical research has pinpointed abatacept as a potential medication alleviating the proatherogenic impacts of ICIs. Observational studies indicate that the proatherosclerotic effects of ICIs might be modulable by statins. However, whether convenient drugs used in cardiovascular disease (CD) are safe to use and efficient in a collective of non-CD patients remains unknown [15]. In a multicenter observational retrospective study, the use of β-blockers, aspirin, and statins was independently related to an increased objective response rate in patients treated with ICIs favoring the use of those drugs [12]. Prospective clinical studies and preclinical models are needed to further support these correlations.
Translating preclinical models to understand ICI-induced cardiotoxicity
Several studies highlighted the similarities between preclinical and clinical findings regarding ICI-mediated cardiotoxicity. Case reports of patients presenting with myocarditis under ICI treatment have shown the infiltration of T cells and macrophages in myocardial tissue [25, 43]. As preclinical models outlined before, overall proinflammatory processes mediated by cytokines and activation of Th17 cells were clinically observed as driving factors in mediating inflammatory processes during ICI treatment, leading to LV dysfunction [18, 23, 44, 56, 74, 89]. Translational research approaches have identified both myosin and troponin as putative autoantigens in the mediation of ICI-induced myocarditis [8, 9, 43]. Autopsies comparing cancer patients who underwent ICI treatment and those who did not receive ICIs showed an altered inflammatory cell composition in coronary artery atherosclerotic plaques. The inflammatory response was predominantly T cell-driven, in line with preclinical findings in Ldlr−/− mice. Given the protracted progression and multifaceted etiology of atherosclerosis, clinical data are necessary to understand the true phenotype [65, 71]. Incorporating cardioprotective strategies like remote ischemic preconditioning, into translational research is essential for a comprehensive understanding [38, 39].
Limitations of preclinical models of ICI-mediated cardiotoxicity
The preclinical models used to assess ICI-induced cardiotoxicity described in this review exhibit various limitations. Knockout models, which eliminate pharmacokinetics of ICIs may poorly mirror the clinical use of disease [55]. Approaches based on the administration of ICIs use murine-specific antibodies at doses that exceed those in clinical practice which may lead to an overestimation or insufficient representation of the effects, thus limiting transfer to patients [3, 27, 30, 93].
The impact of the microbiome and environmental pathogens on the immune system is considered highly important in mediating ICI-related anti-cancer effects, but is inadequately represented in animal models of immune-mediated diseases [36, 81]. Mouse models do not sufficiently cover human heterogeneity, including age, gender, social factors, comorbidities or race [1, 88, 95].
Conclusion
Preclinical models of ICI-mediated cardiotoxicity play a crucial role in understanding and addressing potential cardiovascular side effects associated with this revolutionary cancer therapy. These models often exhibit a predisposing condition together with immune checkpoint blockade or deficiency, suggesting that pre-existing vulnerabilities contribute to cardiac adverse events. CD8+ T cells emerge as the primary contributors to cardiac inflammation following ICI administration. The involvement of other immune cells, such as CD4+ T cells, B cells, monocytes, and Tregs exhibits inconsistency across various studies. Troponin and α-myosin are recognized as potential autoantigens implicated in ICI-related cardiotoxicity but a clinical transfer is missing so far. Preclinical models not only contribute to the comprehension of ICI cardiotoxicity and the exploration of potential therapeutic interventions for managing associated side effects but also offer insights into the influence of immune checkpoints on cardiac integrity in healthy individuals. They provide valuable information regarding the implications of immune checkpoint activity on the progression of cardiac diseases. Future investigations must adopt both bench-to-bedside and bedside-to-bench approaches to bridge the gap between preclinical models and the human pathophysiology of ICI-induced cardiotoxicity. A systematic assessment of cardiovascular complications in oncological studies is crucial for the early identification of complications that must be targeted in future translational research (bedside-to-bench). A close interface with clinical data will enhance the transferability of preclinical findings to the clinic (bench-to-bedside), facilitated using adequate experimental models. These strategies aim to advance diagnostics, therapeutics, and especially preventive measures against ICI-induced cardiotoxicity.
Data availability
All sources and datasets are publicly available and cited within the article.
References
Addison D, Branch M, Baik AH, Fradley MG, Okwuosa T, Reding KW, Simpson KE, Suero-Abreu GA, Yang EH, Yancy CW (2023) Equity in cardio-oncology care and research: a scientific statement from the American Heart Association. Circulation 148:297–308. https://doi.org/10.1161/CIR.0000000000001158
Afanasyeva M, Georgakopoulos D, Rose NR (2004) Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun Rev 3:476–486. https://doi.org/10.1016/j.autrev.2004.08.009
Agrawal S, Feng Y, Roy A, Kollia G, Lestini B (2016) Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer 4:72. https://doi.org/10.1186/s40425-016-0177-2
Alturki NA (2023) Review of the immune checkpoint inhibitors in the context of cancer treatment. J Clin Med 12:4301. https://doi.org/10.3390/jcm12134301
Andres MS, Ramalingam S, Rosen SD, Baksi J, Khattar R, Kirichenko Y, Young K, Yousaf N, Okines A, Huddart R, Harrington K, Furness AJS, Turajlic S, Pickering L, Popat S, Larkin J, Lyon AR (2022) The spectrum of cardiovascular complications related to immune-checkpoint inhibitor treatment: including myocarditis and the new entity of non inflammatory left ventricular dysfunction. Cardio-Oncol Lond Engl 8:21. https://doi.org/10.1186/s40959-022-00147-w
Andrews LP, Marciscano AE, Drake CG, Vignali DAA (2017) LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 276:80–96. https://doi.org/10.1111/imr.12519
Arana Y, Gálvez RI, Jacobs T (2022) Role of the PD-1/PD-L1 pathway in experimental Trypanosoma cruzi infection and potential therapeutic options. Front Immunol 13:866120. https://doi.org/10.3389/fimmu.2022.866120
Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, Tannous E, Zhang Y, Sugiura A, Taylor BC, Hanna A, Zhang S, Amancherla K, Tai W, Wright JJ, Wei SC, Opalenik SR, Toren AL, Rathmell JC, Ferrell PB, Phillips EJ, Mallal S, Johnson DB, Allison JP, Moslehi JJ, Balko JM (2022) T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611:818–826. https://doi.org/10.1038/s41586-022-05432-3
Bockstahler M, Fischer A, Goetzke CC, Neumaier HL, Sauter M, Kespohl M, Müller A-M, Meckes C, Salbach C, Schenk M, Heuser A, Landmesser U, Weiner J, Meder B, Lehmann L, Kratzer A, Klingel K, Katus HA, Kaya Z, Beling A (2020) Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation 141:1885–1902. https://doi.org/10.1161/CIRCULATIONAHA.119.043171
Bu D, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, Jarolim P, Freeman GJ, Sharpe AH, Lichtman AH (2011) Impairment of the PD-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 31:1100–1107. https://doi.org/10.1161/ATVBAHA.111.224709
Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein H-H, Zernecke A (2014) Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS ONE 9:e93280. https://doi.org/10.1371/journal.pone.0093280
Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, Rastelli F, Bisonni R, Santini D, Russano M, Anesi C, Giusti R, Filetti M, Marchetti P, Botticelli A, Gelibter A, Occhipinti MA, Marconcini R, Vitale MG, Nicolardi L, Chiari R, Bareggi C, Nigro O, Tuzi A, De Tursi M, Petragnani N, Pala L, Bracarda S, Macrini S, Inno A, Zoratto F, Veltri E, Di Cocco B, Mallardo D, Vitale MG, Pinato DJ, Porzio G, Ficorella C, Ascierto PA (2020) Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer 8:e001361. https://doi.org/10.1136/jitc-2020-001361
Delombaerde D, Vervloet D, Franssen C, Croes L, Gremonprez F, Prenen H, Peeters M, Vulsteke C (2021) Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open 6:100216. https://doi.org/10.1016/j.esmoop.2021.100216
Dolladille C, Akroun J, Morice P-M, Dompmartin A, Ezine E, Sassier M, Da-Silva A, Plane A-F, Legallois D, L’Orphelin J-M, Alexandre J (2021) Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J 42:4964–4977. https://doi.org/10.1093/eurheartj/ehab618
Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U, Neilan TG (2020) Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142:2299–2311. https://doi.org/10.1161/CIRCULATIONAHA.120.049981
D’Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, Fosbøl E, Køber L, Gustafsson F, Andersson C, Gislason G, Torp-Pedersen C, Schou M (2021) The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 42:1621–1631. https://doi.org/10.1093/eurheartj/ehaa884
Du S, Zhou L, Alexander GS, Park K, Yang L, Wang N, Zaorsky NG, Ma X, Wang Y, Dicker AP, Lu B (2018) PD-1 modulates radiation-induced cardiac toxicity through cytotoxic T lymphocytes. J Thorac Oncol 13:510–520. https://doi.org/10.1016/j.jtho.2017.12.002
Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJA, Hobo W, Gorecka MA, de Haan AFJ, Mulders P, Punt CJA, Jacobs JFM, Schalken JA, Oosterwijk E, van Eenennaam H, Boots AM (2012) PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 35:169. https://doi.org/10.1097/CJI.0b013e318247a4e7
Efentakis P, Choustoulaki A, Kostopoulos Ι, Varela A, Georgoulis A, Tsekenis G, Gakiopoulou C, Ntanasis-Stathopoulos I, Davos C, Tsitsiloni O, Dimopoulos MA, Terpos E, Gavriatopoulou M, Andreadou I (2023) Establishment of an in vivo murine model of immune checkPoint inhibitors cardiotoxicity: emerging role of vascular permeability in pembrolizumab-induced cardiotoxicity. Eur Heart J 44(ehad655):770. https://doi.org/10.1093/eurheartj/ehad655.770
Efentakis P, Choustoulaki A, Kwiatkowski G, Varela A, Kostopoulos IV, Tsekenis G, Ntanasis-Stathopoulos I, Georgoulis A, Vorgias CE, Gakiopoulou H, Briasoulis A, Davos CH, Kostomitsopoulos N, Tsitsilonis O, Dimopoulos MA, Terpos E, Chłopicki S, Gavriatopoulou M, Andreadou I (2024) Early microvascular coronary endothelial dysfunction precedes pembrolizumab-induced cardiotoxicity. Preventive role of high dose of atorvastatin. Basic Res Cardiol. https://doi.org/10.1007/s00395-024-01046-0
Ewing MM, Karper JC, Abdul S, de Jong RCM, Peters HAB, de Vries MR, Redeker A, Kuiper J, Toes REM, Arens R, Jukema JW, Quax PHA (2013) T-cell co-stimulation by CD28–CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol 168:1965–1974. https://doi.org/10.1016/j.ijcard.2012.12.085
Ferdinandy P, Baczkó I, Bencsik P, Giricz Z, Görbe A, Pacher P, Varga ZV, Varró A, Schulz R (2019) Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J 40:1771–1777. https://doi.org/10.1093/eurheartj/ehy365
Finke D, Heckmann MB, Salatzki J, Riffel J, Herpel E, Heinzerling LM, Meder B, Völkers M, Müller OJ, Frey N, Katus HA, Leuschner F, Kaya Z, Lehmann LH (2021) Comparative transcriptomics of immune checkpoint inhibitor myocarditis identifies guanylate binding protein 5 and 6 dysregulation. Cancers 13:2498. https://doi.org/10.3390/cancers13102498
Frodermann V, Nahrendorf M (2018) Macrophages and cardiovascular health. Physiol Rev 98:2523–2569. https://doi.org/10.1152/physrev.00068.2017
Ganatra S, Neilan TG (2018) Immune checkpoint inhibitor-associated myocarditis. Oncologist 23:879–886. https://doi.org/10.1634/theoncologist.2018-0130
Gergely TG, Drobni ZD, Kallikourdis M, Zhu H, Meijers WC, Neilan TG, Rassaf T, Ferdinandy P, Varga ZV (2024) Immune checkpoints in cardiac physiology and pathology: therapeutic targets for heart failure. Nat Rev Cardiol. https://doi.org/10.1038/s41569-023-00986-9
Gergely TG, Kucsera D, Tóth VE, Kovács T, Sayour NV, Drobni ZD, Ruppert M, Petrovich B, Ágg B, Onódi Z, Fekete N, Pállinger É, Buzás EI, Yousif LI, Meijers WC, Radovits T, Merkely B, Ferdinandy P, Varga ZV (2023) Characterization of immune checkpoint inhibitor-induced cardiotoxicity reveals interleukin-17A as a driver of cardiac dysfunction after anti-PD-1 treatment. Br J Pharmacol 180:740–761. https://doi.org/10.1111/bph.15984
Glembotski CC (2011) Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol 51:512–517. https://doi.org/10.1016/j.yjmcc.2010.10.008
Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S (2012) Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem 287:25893–25904. https://doi.org/10.1074/jbc.M112.356345
Goldstein DA, Ratain MJ, Saltz LB (2020) Weight-based dosing of pembrolizumab every 6 weeks in the time of COVID-19. JAMA Oncol 6:1694–1695. https://doi.org/10.1001/jamaoncol.2020.2493
Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH (2007) Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Investig 117:2974–2982. https://doi.org/10.1172/JCI31344
Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH (2003) IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Investig 111:671–680. https://doi.org/10.1172/JCI200316867
Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH (2007) Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell-mediated injury in the heart. Circulation 116:2062–2071. https://doi.org/10.1161/CIRCULATIONAHA.107.709360
Grievink HW, Smit V, Verwilligen RAF, Bernabé Kleijn MNA, Smeets D, Binder CJ, Yagita H, Moerland M, Kuiper J, Bot I, Foks AC (2021) Stimulation of the PD-1 pathway decreases atherosclerotic lesion development in Ldlr deficient mice. Front Cardiovasc Med 8:740531. https://doi.org/10.3389/fcvm.2021.740531
Gutierrez FRS, Mariano FS, Oliveira CJF, Pavanelli WR, Guedes PMM, Silva GK, Campanelli AP, Milanezi CM, Azuma M, Honjo T, Teixeira MM, Aliberti JCS, Silva JS (2011) Regulation of Trypanosoma cruzi-induced myocarditis by programmed death cell receptor 1. Infect Immun 79:1873–1881. https://doi.org/10.1128/IAI.01047-10
Hansen AK, Hansen CHF (2021) The microbiome and rodent models of immune mediated diseases. Mamm Genome 32:251–262. https://doi.org/10.1007/s00335-021-09866-4
Herrmann J (2020) Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 17:474–502. https://doi.org/10.1038/s41569-020-0348-1
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Heusch G (2023) Cardioprotection in cardio-oncology: a case for concern? Cardiovasc Res 119:e144–e145. https://doi.org/10.1093/cvr/cvad111
Huang YV, Lee D, Sun Y, Chou H, Xu B, Lin Z, Branche C, Bayer A, Waliany S, Neal J, Wakelee H, Witteles R, Nguyen P, Graves E, Alcaide P, Berry GJ, Wu SM, Zhu H (2024) A novel therapeutic approach using CXCR3 blockade to treat immune checkpoint inhibitor-mediated myocarditis. https://doi.org/10.1101/2024.01.30.576279
Huo J-L, Wang Y-T, Fu W-J, Lu N, Liu Z-S (2022) The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol 13:956090
Ji C, Roy MD, Golas J, Vitsky A, Ram S, Kumpf SW, Martin M, Barletta F, Meier WA, Hooper AT, Sapra P, Khan NK, Finkelstein M, Guffroy M, Buetow BS (2019) Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res 25:4735–4748. https://doi.org/10.1158/1078-0432.CCR-18-4083
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749–1755. https://doi.org/10.1056/NEJMoa1609214
Kim ST, Bittar M, Kim HJ, Neelapu SS, Zurita AJ, Nurieva R, Suarez-Almazor ME (2019) Recurrent pseudogout after therapy with immune checkpoint inhibitors: a case report with immunoprofiling of synovial fluid at each flare. J Immunother Cancer 7:126. https://doi.org/10.1186/s40425-019-0597-x
Klocke K, Sakaguchi S, Holmdahl R, Wing K (2016) Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A 113:E2383–E2392. https://doi.org/10.1073/pnas.1603892113
Lafuse WP, Wozniak DJ, Rajaram MVS (2020) Role of cardiac macrophages on cardiac inflammation. Fibros Tissue Repair Cells 10:51. https://doi.org/10.3390/cells10010051
Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP, Dempsey PJ, Workman CJ, Vignali DAA (2007) Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J 26:494–504. https://doi.org/10.1038/sj.emboj.7601520
Liu Y, Chen Y, Zeng Z, Liu A (2022) Arrhythmic events associated with immune checkpoint inhibitors therapy: a real-world study based on the Food and Drug Administration Adverse Event Reporting System database. Cancer Med 12:6637–6648. https://doi.org/10.1002/cam4.5438
Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A (2007) CTLA-4 ablation and interleukin-12-driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 101:248–257. https://doi.org/10.1161/CIRCRESAHA.106.147124
Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelly VR (2008) PD-L1 Regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol Baltim Md 1950 181:2513–2521
Ma K, Lv S, Liu B, Liu Z, Luo Y, Kong W, Xu Q, Feng J, Wang X (2013) CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE−/− mice. Cardiovasc Res 97:349–359. https://doi.org/10.1093/cvr/cvs330
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755–1764. https://doi.org/10.1016/j.jacc.2018.02.037
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16:563–580. https://doi.org/10.1038/s41571-019-0218-0
Matsumoto T, Sasaki N, Yamashita T, Emoto T, Kasahara K, Mizoguchi T, Hayashi T, Yodoi K, Kitano N, Saito T, Yamaguchi T, Hirata K-I (2016) Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol 36:1141–1151. https://doi.org/10.1161/ATVBAHA.115.306848
Meng C, Fan L, Wang X, Wang Y, Li Y, Pang S, Lv S, Zhang J (2022) Preparation and evaluation of animal models of cardiotoxicity in antineoplastic therapy. Oxid Med Cell Longev 2022:e3820591. https://doi.org/10.1155/2022/3820591
Michel L, Helfrich I, Hendgen-Cotta UB, Mincu R-I, Korste S, Mrotzek SM, Spomer A, Odersky A, Rischpler C, Herrmann K, Umutlu L, Coman C, Ahrends R, Sickmann A, Löffek S, Livingstone E, Ugurel S, Zimmer L, Gunzer M, Schadendorf D, Totzeck M, Rassaf T (2022) Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J 43:316–329. https://doi.org/10.1093/eurheartj/ehab430
Michel L, Hendgen-Cotta UB, Mincu RI, Helfrich I, Korste S, Mrotzek SM, Rischpler C, Herrmann K, Ugurel S, Zimmer L, Coman C, Ahrends R, Schadendorf D, Rassaf T, Totzeck M (2020) Preclinical and clinical assessment of immune checkpoint inhibitor-associated left ventricular dysfunction. Eur Heart J 41(ehaa946):3260. https://doi.org/10.1093/ehjci/ehaa946.3260
Michel L, Korste S, Spomer A, Hendgen-Cotta UB, Rassaf T, Totzeck M (2022) PD1 deficiency modifies cardiac immunity during baseline conditions and in reperfused acute myocardial infarction. Int J Mol Sci 23:7533. https://doi.org/10.3390/ijms23147533
Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O (2006) Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer Oxf Engl 1990 54:139–148. https://doi.org/10.1016/j.ejca.2015.11.016
Moradi A, Kodali A, Okoye C, Klein DH, Mohamoud I, Olanisa OO, Parab P, Chaudhary P, Mukhtar S, Mohammed L (2023) A systematic review of myocarditis induced by immune checkpoint inhibitors: how concerning is the most common cardiotoxicity of immune checkpoint inhibitors? Cureus 15:e42071. https://doi.org/10.7759/cureus.42071
Mrotzek SM, Rassaf T, Totzeck M (2020) Cardiovascular damage associated with chest irradiation. Front Cardiovasc Med 7:41. https://doi.org/10.3389/fcvm.2020.00041
Mulholland M, Kritikou E, Katra P, Nilsson J, Björkbacka H, Lichtman AH, Rodriguez A, Engelbertsen D (2022) LAG3 regulates T cell activation and plaque infiltration in atherosclerotic mice. JACC CardioOncol 4:635–645. https://doi.org/10.1016/j.jaccao.2022.09.005
Mullick N, Nambudiri VE (2023) Relatlimab-nivolumab: a practical overview for dermatologists. J Am Acad Dermatol 89:1031–1037. https://doi.org/10.1016/j.jaad.2023.06.024
Myers CJ, Lu B (2017) Decreased survival after combining thoracic irradiation and an anti-PD-1 antibody is correlated with increased T cell infiltration into cardiac and lung tissues. Int J Radiat Oncol Biol Phys 99:1129–1136. https://doi.org/10.1016/j.ijrobp.2017.06.2452
Newman JL, Stone JR (2019) Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc Pathol 43:107148. https://doi.org/10.1016/j.carpath.2019.107148
Nicolás-Ávila JA, Pena-Couso L, Muñoz-Cánoves P, Hidalgo A (2022) Macrophages, metabolism and heterophagy in the heart. Circ Res 130:418–431. https://doi.org/10.1161/CIRCRESAHA.121.319812
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322. https://doi.org/10.1126/science.291.5502.319
Okazaki T, Okazaki I, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T (2011) PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 208:395–407. https://doi.org/10.1084/jem.20100466
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T (2003) Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 9:1477–1483. https://doi.org/10.1038/nm955
Ottaviano M, De Placido S, Ascierto PA (2019) Recent success and limitations of immune checkpoint inhibitors for cancer: a lesson from melanoma. Virchows Arch 474:421–432. https://doi.org/10.1007/s00428-019-02538-4
Poels K, van Leent MMT, Boutros C, Tissot H, Roy S, Meerwaldt AE, Toner YCA, Reiche ME, Kusters PJH, Malinova T, Huveneers S, Kaufman AE, Mani V, Fayad ZA, de Winther MPJ, Marabelle A, Mulder WJM, Robert C, Seijkens TTP, Lutgens E (2020) Immune checkpoint inhibitor therapy aggravates T cell-driven plaque inflammation in atherosclerosis. JACC CardioOncol 2:599–610. https://doi.org/10.1016/j.jaccao.2020.08.007
Poels K, van Leent MMT, Reiche ME, Kusters PJH, Huveneers S, de Winther MPJ, Mulder WJM, Lutgens E, Seijkens TTP (2020) Antibody-mediated inhibition of CTLA4 aggravates atherosclerotic plaque inflammation and progression in hyperlipidemic mice. Cells 9:1987. https://doi.org/10.3390/cells9091987
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126. https://doi.org/10.1056/NEJM199901143400207
Roth ME, Muluneh B, Jensen BC, Madamanchi C, Lee CB (2016) Left ventricular dysfunction after treatment with ipilimumab for metastatic melanoma. Am J Ther 23:e1925–e1928. https://doi.org/10.1097/MJT.0000000000000430
Rowshanravan B, Halliday N, Sansom DM (2018) CTLA-4: a moving target in immunotherapy. Blood 131:58–67. https://doi.org/10.1182/blood-2017-06-741033
Rubio-Infante N, Ramírez-Flores YA, Castillo EC, Lozano O, García-Rivas G, Torre-Amione G (2021) Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail 23:1739–1747. https://doi.org/10.1002/ejhf.2289
Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M (2016) Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 119:853–864. https://doi.org/10.1161/CIRCRESAHA.116.309001
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867. https://doi.org/10.1016/0092-8674(92)90029-c
Taleb S (2016) Inflammation in atherosclerosis. Arch Cardiovasc Dis 109:708–715. https://doi.org/10.1016/j.acvd.2016.04.002
Tao L, Reese TA (2017) Making mouse models that reflect human immune responses. Trends Immunol 38:181–193. https://doi.org/10.1016/j.it.2016.12.007
Tarrio ML, Grabie N, Bu D, Sharpe AH, Lichtman AH (2012) PD-1 protects against inflammation and myocyte damage in T cell mediated myocarditis. J Immunol Baltim Md 1950 188:4876–4884. https://doi.org/10.4049/jimmunol.1200389
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–547. https://doi.org/10.1016/1074-7613(95)90125-6
Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T (2019) Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 280:163–175. https://doi.org/10.1016/j.ijcard.2019.01.038
Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4:1721–1728. https://doi.org/10.1001/jamaoncol.2018.3923
Wang J, Okazaki I-M, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T (2010) PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 22:443–452. https://doi.org/10.1093/intimm/dxq026
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–988. https://doi.org/10.1126/science.270.5238.985
Wei SC, Meijers WC, Axelrod ML, Anang N-AAS, Screever EM, Wescott EC, Johnson DB, Whitley E, Lehmann L, Courand P-Y, Mancuso JJ, Himmel LE, Lebrun-Vignes B, Wleklinski MJ, Knollmann BC, Srinivasan J, Li Y, Atolagbe OT, Rao X, Zhao Y, Wang J, Ehrlich LIR, Sharma P, Salem J-E, Balko JM, Moslehi JJ, Allison JP (2021) A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 11:614–625. https://doi.org/10.1158/2159-8290.CD-20-0856
Westdorp H, Sweep MWD, Gorris MAJ, Hoentjen F, Boers-Sonderen MJ, van der Post RS, van den Heuvel MM, Piet B, Boleij A, Bloemendal HJ, de Vries IJM (2021) Mechanisms of immune checkpoint inhibitor-mediated colitis. Front Immunol 12:768957. https://doi.org/10.3389/fimmu.2021.768957
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271–275. https://doi.org/10.1126/science.1160062
Won T, Kalinoski HM, Wood MK, Hughes DM, Jaime CM, Delgado P, Talor MV, Lasrado N, Reddy J, Čiháková D (2022) Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. https://doi.org/10.1016/j.celrep.2022.111611
Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano D, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DAA (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T cell function to promote tumoral immune escape. Cancer Res 72:917–927. https://doi.org/10.1158/0008-5472.CAN-11-1620
Xia W, Zou C, Chen H, Xie C, Hou M (2020) Immune checkpoint inhibitor induces cardiac injury through polarizing macrophages via modulating microRNA-34a/Kruppel-like factor 4 signaling. Cell Death Dis 11:575. https://doi.org/10.1038/s41419-020-02778-2
Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J (2010) Cutting edge: CTLA-4–B7 interaction suppresses Th17 cell differentiation. J Immunol Baltim Md 1950 185:1375–1378. https://doi.org/10.4049/jimmunol.0903369
Zamami Y, Niimura T, Okada N, Koyama T, Fukushima K, Izawa-Ishizawa Y, Ishizawa K (2019) Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol 5:1635–1637. https://doi.org/10.1001/jamaoncol.2019.3113
Zan H, Zhang J, Ardeshna S, Xu Z, Park S-R, Casali P (2009) Lupus-prone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity 42:89–103. https://doi.org/10.1080/08916930802629554
Zhang PL, Lun M, Teng J, Huang J, Blasick TM, Yin L, Herrera GA, Cheung JY (2004) Preinduced molecular chaperones in the endoplasmic reticulum protect cardiomyocytes from lethal injury. Ann Clin Lab Sci 34:449–457
Zhang Y, Sun C, Li Y, Qin J, Amancherla K, Jing Y, Hu Q, Liang K, Zhang Z, Ye Y, Huang LA, Nguyen TK, Egranov SD, Zhao Z, Wu A, Xi Y, Yao J, Hung M-C, Calin GA, Cheng J, Lim B, Lehmann LH, Salem J-E, Johnson DB, Curran MA, Yu D, Han L, Darabi R, Yang L, Moslehi JJ, Lin C (2022) Hormonal therapies upregulate MANF and overcome female susceptibility to immune checkpoint inhibitor-myocarditis. Sci Transl Med 14:eabo1981. https://doi.org/10.1126/scitranslmed.abo1981
Funding
Open Access funding enabled and organized by Projekt DEAL. T.R. was supported by the Deutsche Forschungsgemeinschaft (RA 969/12-1). U.B.H.-C. was supported by the Deutsche Forschungsgemeinschaft (HE 6317/2-1). L.M. was supported by the IFORES scholarship of the Medical Faculty, University Duisburg-Essen, Germany.
Author information
Authors and Affiliations
Contributions
Idea for the article: T.R. and L.M.; literature search: F.B.; data analysis and writing: F.B., L.M.; design of figures: F.B.; revision of work: J.V., T.L., M.T., U.B.H.-C.; final approval: all the authors.
Corresponding author
Ethics declarations
Conflict of interest
F.B. and T.L. have no competing interests to declare that are relevant to the content of this article. M.T. reports personal fees and others from Edwards, Novartis, Bristol Myers Squibb, Bayer, Daiichi Sankyo und AstraZeneca and Pfizer outside of the submitted work. J.V. reports personal fees from Eli Lilly outside of the submitted work. T.R. reports personal fees and others from Edwards, Novartis, Bristol Myers Squibb, Bayer, Daiichi Sankyo und AstraZeneca and Pfizer outside of the submitted work. T.R. is a co-founder of Bimyo, a company focusing on the development of cardioprotective peptides. U.B.H.-C. is a co-founder of Bimyo, a company focusing on the development of cardioprotective peptides. L.M. reports personal fees from Bayer, Alnylam, AstraZeneca, Bristol Myers Squibb, Pfizer, IFFM e.V. and from Bund der Niedergelassenen Kardiologen (BNK) outside of the submitted work.
Ethical approval
This review article is based on existing literature and does not involve any original human or animal data. As such, ethical approval and informed consent are not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buehning, F., Lerchner, T., Vogel, J. et al. Preclinical models of cardiotoxicity from immune checkpoint inhibitor therapy. Basic Res Cardiol 120, 171–185 (2025). https://doi.org/10.1007/s00395-024-01070-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-024-01070-0