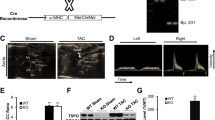
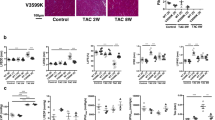
Abstract
The histidine-rich Ca2+-binding protein (HRC) is located in the lumen of the sarcoplasmic reticulum (SR) and exhibits high-capacity Ca2+-binding properties. Overexpression of HRC in the heart resulted in impaired SR Ca2+ uptake and depressed relaxation through its interaction with SERCA2a. However, the functional significance of HRC in overall regulation of calcium cycling and contractility is not currently well defined. To further elucidate the role of HRC in vivo under physiological and pathophysiological conditions, we generated and characterized HRC-knockout (KO) mice. The KO mice were morphologically and histologically normal compared to wild-type (WT) mice. At the cellular level, ablation of HRC resulted in significantly enhanced contractility, Ca2+ transients, and maximal SR Ca2+ uptake rates in the heart. However, after-contractions were developed in 50 % of HRC-KO cardiomyocytes, compared to 11 % in WT mice under stress conditions of high-frequency stimulation (5 Hz) and isoproterenol application. A parallel examination of the electrical activity revealed significant increases in the occurrence of Ca2+ spontaneous SR Ca2+ release and delayed afterdepolarizations with ISO in HRC-KO, compared to WT cells. The frequency of Ca2+ sparks was also significantly higher in HRC-KO cells with ISO, consistent with the elevated SR Ca2+ load in the KO cells. Furthermore, HRC-KO cardiomyocytes showed significantly deteriorated cell contractility and Ca2+-cycling caused possibly by depressed SERCA2a expression after transverse-aortic constriction (TAC). Also HRC-null mice exhibited severe cardiac hypertrophy, fibrosis, pulmonary edema and decreased survival after TAC. Our results indicate that ablation of HRC is associated with poorly regulated SR Ca2+-cycling, and severe pathology under pressure-overload stress, suggesting an essential role of HRC in maintaining the integrity of cardiac function.









Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Arvanitis DA, Sanoudou D, Kolokathis F, Vafiadaki E, Papalouka V, Kontrogianni-Konstantopoulos A, Theodorakis GN, Paraskevaidis IA, Adamopoulos S, Dorn GW 2nd, Kremastinos DT, Kranias EG (2008) The Ser96Ala variant in histidine-rich calcium-binding protein is associated with life-threatening ventricular arrhythmias in idiopathic dilated cardiomyopathy. Eur Heart J 29:2514–2525. doi:10.1093/eurheartj/ehn328
Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG (2007) Histidine-rich Ca2+-binding protein interacts with sarcoplasmic reticulum Ca2+–ATPase. Am J Physiol Heart Circ Physiol 293:H1581–H1589. doi:10.1152/ajpheart.00278.2007
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. doi:10.1146/annurev.physiol.70.113006.100455
Bers DM, Guo T (2005) Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci 1047:86–98. doi:10.1196/annals.1341.008
Brittsan AG, Kranias EG (2000) Phospholamban and cardiac contractile function. J Mol Cell Cardiol 32:2131–2139. doi:10.1006/jmcc.2000.1270
Cha H, Kim JM, Oh JG, Jeong MH, Park CS, Park J, Jeong HJ, Park BK, Lee YH, Jeong D, Yang DK, Bernecker OY, Kim DH, Hajjar RJ, Park WJ (2008) PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility. J Mol Cell Cardiol 45(6):796–803. doi:10.1016/j.yjmcc.2008.09.124
Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD (2009) Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci USA 106:7636–7641. doi:10.1073/pnas.0902919106
Elliott EB, Hasumi H, Otani N, Matsuda T, Matsuda R, Kaneko N, Smith GL, Loughrey CM (2011) K201 (JTV-519) alters the spatiotemporal properties of diastolic Ca2+ release and the associated diastolic contraction during β-adrenergic stimulation in rat ventricular cardiomyocytes. Basic Res Cardiol 106:1009–1022. doi:10.1007/s00395-011-0218-4
Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG (2004) Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol 287:H1705–H1711. doi:10.1152/ajpheart.01211.2003
Florea S, Anjak A, Cai WF, Qian J, Vafiadaki E, Figueria S, Haghighi K, Rubinstein J, Lorenz J, Kranias EG (2012) Constitutive phosphorylation of inhibitor-1 at Ser67 and Thr75 depresses calcium cycling in cardiomyocytes and leads to remodeling upon aging. Basic Res Cardiol 107:279. doi:10.1007/s00395-012-0279-z
Ginsburg KS, Bers DM (2004) Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and ICa trigger. J Physiol 556:463–480. doi:10.1113/jphysiol.2003.055384
Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, Park WJ, Dorn GW 2nd, Bers DM, Kranias EG (2006) Histidine-rich Ca2+ binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol 40:653–665. doi:10.1016/j.yjmcc.2006.02.003
Györke I, Hester N, Jones LR, Györke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86(4):2121–1218. doi:10.1016/S0006-3495(04)74271-X
Györke S, Terentyev D (2008) Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77:245–255. doi:10.1093/cvr/cvm038
Hasenfuss G (1998) Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 37:279–289. doi:10.1016/S0008-6363(97)00277-0
Heinzel FR, Luo Y, Dodoni G, Boengler K, Petrat F, Di Lisa F, de Groot H, Schulz R, Heusch G (2006) Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc Res 71(2):374–382. doi:10.1016/j.cardiores.2006.05.014
Heusch G (2011) Heart rate and heart failure. Circ J 75:229–236
Hofmann SL, Brown MS, Lee E, Pathak RK, Anderson RG, Goldstein JL (1989) Purification of a sarcoplasmic reticulum protein that binds Ca2+ and plasma lipoproteins. J Biol Chem 264:8260–8270
Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS (1989) Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem 264:18083–18090
Hofmann SL, Topham M, Hsieh CL, Francke U (1991) cDNA and genomic cloning of HRC, a human sarcoplasmic reticulum protein, and localization of the gene to human chromosome 19 and mouse chromosome 7. Genomics 9:656–669. doi:10.1016/0888-7543(91)90359-M
Hong S, Kim TW, Choi I, Woo JM, Oh J, Park WJ, Kim DH, Cho C (2005) Complementary DNA cloning, genomic characterization and expression analysis of a mammalian gene encoding histidine-rich calcium binding protein. Biochim Biophys Acta 1727:188–196. doi:10.1016/j.bbaexp.2005.01.006
Jaehnig EJ, Heidt AB, Greene SB, Cornelissen I, Black BL (2006) Increased susceptibility to isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein. Mol Cell Biol 26:9315–9326. doi:10.1128/MCB.00482-06
Kim E, Shin DW, Hong CS, Jeong D, Kim DH, Park WJ (2003) Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein). Biochem Biophys Res Commun 300:192–196. doi:10.1016/S0006-291X(02)02829-2
Kranias EG, Bers DM (2007) Calcium and cardiomyopathies. Subcell Biochem 45:523–537. doi:10.1007/978-1-4020-6191-2_20
Lee HG, Kang H, Kim DH, Park WJ (2001) Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem 276:39533–39538. doi:10.1074/jbc.M010664200
Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers DM, Brown JH (2009) Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest 119:1230–1240. doi:10.1172/JCI38022
Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG (1994) Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 75:401–409. doi:10.1161/01.RES.75.3.401
Oh JG, Jeong D, Cha H, Kim JM, Lifirsu E, Kim J, Yang DK, Park CS, Kho C, Park S, Yoo YJ, Kim DH, Kim J, Hajjar RJ, Park WJ (2012) PICOT increases cardiac contractility by inhibiting PKCζ activity. J Mol Cell Cardiol 53(1):53–63. doi:10.1016/j.yjmcc.2012.03.005
Oh JG, Kim J, Jang SP, Nguen M, Yang DK, Jeong D, Park ZY, Park SG, Hajjar RJ, Park WJ (2013) Decoy peptides targeted to protein phosphatase 1 inhibit dephosphorylation of phospholamban in cardiomyocytes. J Mol Cell Cardiol 56:63–71. doi:10.1016/j.yjmcc.2012.12.005
Pagani ED, Solaro RJ (1984) Coordination of cardiac myofibrillar and sarcotubular activities in rats exercised by swimming. Am J Physiol 247:H909–H915
Park CS, Cha H, Kwon EJ, Jeong D, Hajjar RJ, Kranias EG, Cho C, Park WJ, Kim DH (2012) AAV-mediated knock-down of HRC exacerbates transverse aorta constriction-induced heart failure. PLoS ONE 7(8):e43282. doi:10.1371/journal.pone.0043282
Piacentino V 3rd, Weber CR, Gaughan JP, Margulies KB, Bers DM, Houser SR (2002) Modulation of contractility in failing human myocytes by reverse-mode Na+/Ca2+ exchange. Ann N Y Acad Sci 976:466–471. doi:10.1111/j.1749-6632.2002.tb04776.x
Picello E, Damiani E, Margreth A (1992) Low-affinity Ca2+-binding sites versus Zn2+-binding sites in histidine-rich Ca2+-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem Biophys Res Commun 186:659–667. doi:10.1016/0006-291X(92)90797-O
Puglisi JL, Bassani RA, Bassani JW, Amin JN, Bers DM (1996) Temperature and relative contributions of Ca2+ transport systems in cardiac myocyte relaxation. Am J Physiol 270:H1772–H1778
Ramirez RJ, Sah R, Liu J, Rose RA, Backx PH (2011) Intracellular [Na+] modulates synergy between Na+/Ca2+ exchanger and L-type Ca2+ current in cardiac excitation-contraction coupling during action potentials. Basic Res Cardiol 106:967–977. doi:10.1007/s00395-011-0202-z
Ridgeway AG, Petropoulos H, Siu A, Ball JK, Skerjanc IS (1999) Cloning, tissue distribution, subcellular localization and overexpression of murine histidine-rich Ca2+ binding protein. FEBS Lett 456:399–402. doi:10.1016/S0014-5793(99)00993-X
Shah AM, Sauer H (2006) Transmitting biological information using oxygen: reactive oxygen species as signalling molecules in cardiovascular pathophysiology. Cardiovasc Res 71:191–194. doi:10.1016/j.cardiores.2006.05.018
Solaro RJ, Briggs FN (1974) Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circ Res 34:531–540. doi:10.1161/01.RES.34.4.531
Sossalla S, Maurer U, Schotola H, Hartmann N, Didié M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS (2011) Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res Cardiol 106:263–272. doi:10.1007/s00395-010-0136-x
Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Györke S (2008) Modulation of SR Ca2+ release by luminal Ca2+ and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J 95:2037–2048. doi:10.1529/biophysj.107.128249
Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Györke I, Terentyeva R, Williams SC, Györke S (2005) Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res 96:651–658. doi:10.1161/01.RES.0000160609.98948.25
Toischer K, Lehnart SE, Tenderich G, Milting H, Körfer R, Schmitto JD, Schöndube FA, Kaneko N, Loughrey CM, Smith GL, Hasenfuss G, Seidler T (2010) K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol 105:279–287. doi:10.1007/s00395-009-0057-8
van Heeswijk MP, Geertsen JA, van Os CH (1984) Kinetic properties of the ATP-dependent Ca2+ pump and the Na+/Ca2+ exchange system in basolateral membranes from rat kidney cortex. J Membr Biol 79:19–31. doi:10.1007/BF01868523
Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Györke S (2004) Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res 94:471–477. doi:10.1161/01.RES.0000115944.10681.EB
Vinet L, Pezet M, Bito V, Briec F, Biesmans L, Rouet-Benzineb P, Gellen B, Prévilon M, Chimenti S, Vilaine JP, Charpentier F, Sipido KR, Mercadier JJ (2012) Cardiac FKBP12.6 overexpression protects against triggered ventricular tachycardia in pressure overloaded mouse hearts. Basic Res Cardiol 107:246. doi:10.1007/s00395-012-0246-8
Wang HS, Cohen IS (2003) Calcium channel heterogeneity in canine left ventricular myocytes. J Physiol 547:825–833. doi:10.1113/jphysiol.2002.035410
Wehrens XH (2007) Leaky ryanodine receptors cause delayed afterdepolarizations and ventricular arrhythmias. Eur Heart J 28:1054–1056. doi:10.1093/eurheartj/ehm068
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272:23389–23397. doi:10.1074/jbc.272.37.23389
Zhao W, Yuan Q, Qian J, Waggoner JR, Pathak A, Chu G, Mitton B, Sun X, Jin J, Braz JC, Hahn HS, Marreez Y, Syed F, Pollesello P, Annila A, Wang HS, Schultz Jel J, Molkentin JD, Liggett SB, Dorn GW 2nd, Kranias EG (2006) The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca2+–ATPase superinhibition and cardiac remodeling. Circulation 113:995–1004. doi:10.1161/CIRCULATIONAHA.105.583351
Zhou X, Fan GC, Ren X, Waggoner JR, Gregory KN, Chen G, Jones WK, Kranias EG (2007) Overexpression of histidine-rich Ca2+-binding protein protects against ischemia/reperfusion-induced cardiac injury. Cardiovasc Res 75:487–497. doi:10.1016/j.cardiores.2007.04.005
Acknowledgments
This work was supported by Korean Systems Biology Research Grant M10503010001-06N0301-00110 (to D.H.K. and C.C.); GIST Systems Biology Infrastructure Establishment Grant (to D.H.K. and C.C.); National Institutes of Health Grants HL-48093 (to C.F.A), HL-26507 (to E.G.K.), HL-64018 and HL-77101 (to E.G.K.).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
C.S. Park, S. Chen and H. Lee contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, C.S., Chen, S., Lee, H. et al. Targeted ablation of the histidine-rich Ca2+-binding protein (HRC) gene is associated with abnormal SR Ca2+-cycling and severe pathology under pressure-overload stress. Basic Res Cardiol 108, 344 (2013). https://doi.org/10.1007/s00395-013-0344-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0344-2