Abstract
Purpose
The human obesity susceptibility gene, FTO, associates with body mass and obesity in humans through regulation of energy expenditure and intake. We aimed to determine how fatty acids in plasma and in diet associate with FTO gene expression in subcutaneous and visceral adipose tissues.
Methods
In this study, 97 participants aged ≥ 18 years were selected from patients admitted to the hospital for abdominal surgeries. Habitual dietary intake of participants was collected using a valid and reliable food frequency questionnaire (FFQ), from which the intake of fatty acids was quantified. Plasma fatty acids were assessed by gas–liquid chromatography. The mRNA expression of the FTO gene in visceral and subcutaneous adipose tissues obtained by biopsy was measured by Real-Time Quantitative Reverse Transcription PCR. Standardized β-coefficients were calculated by multivariable linear regression.
Results
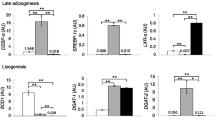
After adjusting for age, homeostasis model insulin resistance index (HOMA-IR), and body mass index, total fatty acid intake was significantly associated with FTO gene expression in visceral (STZβ = 0.208, P = 0.037) and subcutaneous (STZβ = 0.236, P = 0.020) adipose tissues. Dietary intake of monounsaturated fatty acid (MUFA) and polyunsaturated fatty acids (PUFA) had positive significant associations with the expression of FTO in visceral (STZβ = 0.227, P = 0.023; STZβ = 0.346, P < 0.001, respectively) and subcutaneous (STZβ = 0.227, P = 0.026; STZβ = 0.274, P = 0.006, respectively) adipose tissues. There were no associations between plasma fatty acids and FTO mRNA expression in either subcutaneous or visceral adipose tissues.
Conclusion
The weak association of dietary total fatty acids, MUFA, and PUFA with FTO gene expression in both adipose tissues may highlight the importance of dietary fatty acids composition along with total fat intake in relation to FTO gene expression.

Similar content being viewed by others
Data availability
The data set is the property of Research Institute for Endocrine Sciences (RIES) and is made available upon approval of the research proposal by the research council and the ethics committee. The RIES ethics committee must issue an approval in case of a request for access to the de-identified dataset. Data request may be sent to the head of the RIES Ethics Committee Dr. Azita Zadeh-Vakili at email: azitavakili@endocrine.ac.ir.
References
Al-Attar SA, Pollex RL, Ban MR, Young TK, Bjerregaard P, Anand SS, Yusuf S, Zinman B, Harris SB, Hanley AJ (2008) Association between the FTO rs9939609 polymorphism and the metabolic syndrome in a non-Caucasian multi-ethnic sample. Cardiovasc Diabetol 7(1):5
Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39(6):724
Frayling TM (2007) Genome–wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 8(9):657
Mizuno T (2018) Fat mass and obesity associated (FTO) gene and hepatic glucose and lipid metabolism. Nutrients 10(11):1600
Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR (2007) Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3(7):e115. https://doi.org/10.1371/journal.pgen.0030115
Grunnet LG, Brøns C, Jacobsen S, Nilsson E, Astrup A, Hansen T, Pedersen O, Poulsen P, Quistorff B, Vaag A (2009) Increased recovery rates of phosphocreatine and inorganic phosphate after isometric contraction in oxidative muscle fibers and elevated hepatic insulin resistance in homozygous carriers of the A-allele of FTO rs9939609. J Clin Endocrinol Metab 94(2):596–602. https://doi.org/10.1210/jc.2008-1592
Vasan SK, Karpe F, Gu HF, Brismar K, Fall CH, Ingelsson E, Fall T (2014) FTO genetic variants and risk of obesity and type 2 diabetes: a meta-analysis of 28,394 Indians. Obesity 22(3):964–970. https://doi.org/10.1002/oby.20606
Yeo GS (2014) The role of the FTO (Fat Mass and Obesity Related) locus in regulating body size and composition. Mol Cell Endocrinol 397(1–2):34–41. https://doi.org/10.1016/j.mce.2014.09.012
Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL (2008) Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 294(4):R1185–R1196
Gerken T, Girard CA, Tung Y-CL, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318(5855):1469–1472
Zabena C, González-Sánchez JL, Martínez-Larrad MT, Torres-García A, Alvarez-Fernández-Represa J, Corbatón-Anchuelo A, Pérez-Barba M, Serrano-Ríos M (2009) The FTO obesity gene. Genotyping and gene expression analysis in morbidly obese patients. Obes Surg 19(1):87–95
Lappalainen T, Kolehmainen M, Schwab U, Pulkkinen L, de Mello VD, Vaittinen M, Laaksonen DE, Poutanen K, Uusitupa M, Gylling H (2010) Gene expression of FTO in human subcutaneous adipose tissue, peripheral blood mononuclear cells and adipocyte cell line. J Nutrigenetics Nutrigenomics 3(1):37–45
Grunnet LG, Nilsson E, Ling C, Hansen T, Pedersen O, Groop L, Vaag A, Poulsen P (2009) Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes 58(10):2402–2408
Zhao X, Yang Y, Sun B-F, Shi Y, Yang X, Xiao W, Hao Y-J, Ping X-L, Chen Y-S, Wang W-J (2014) FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24(12):1403
Wåhlén K, Sjölin E, Hoffstedt J (2008) The common rs9939609 gene variant of the fat mass-and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res 49(3):607–611
Do R, Bailey SD, Desbiens K, Belisle A, Montpetit A, Bouchard C, Pérusse L, Vohl M-C, Engert JC (2008) Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes 57(4):1147–1150
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci AMS 9(2):191
Yuzbashian E, Zarkesh M, Asghari G, Hedayati M, Safarian M, Mirmiran P, Khalaj A (2018) Is apelin gene expression and concentration affected by dietary intakes? A systematic review. Crit Rev Food Sci Nutr 58(4):680–688. https://doi.org/10.1080/10408398.2016.1262325
Yuzbashian E, Asghari G, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A (2019a) The association of dietary carbohydrate with FTO gene expression in visceral and subcutaneous adipose tissue of adults without diabetes. Nutrition 63–64:92–97. https://doi.org/10.1016/j.nut.2018.12.014
Yuzbashian E, Asghari G, Aghayan M, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A (2019) Dietary glycemic index and dietary glycemic load is associated with apelin gene expression in visceral and subcutaneous adipose tissues of adults. Nutr metab 16(1):1–9
Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Bruning JC, Nolan PM, Ashcroft FM, Cox RD (2010) Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 42(12):1086–1092. https://doi.org/10.1038/ng.713
Berraondo B, Martinez JA (2000) Free fatty acids are involved in the inverse relationship between hormone-sensitive lipase (HSL) activity and expression in adipose tissue after high-fat feeding or beta3-adrenergic stimulation. Obes Res 8(3):255–261. https://doi.org/10.1038/oby.2000.30
Greco AV, Mingrone G, Vettor R, Manco M, Rosa G, Capristo E, Federspil G, Castagneto M, Gasbarrini G (2002) Lowering of circulating free-fatty acids levels and reduced expression of leptin in white adipose tissue in postobesity status. J Investig Med 50(3):207–213. https://doi.org/10.2310/6650.2002.33435
Asghari G, Rezazadeh A, Hosseini-Esfahani F, Mehrabi Y, Mirmiran P, Azizi F (2012) Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br J Nutr 108(6):1109–1117. https://doi.org/10.1017/s0007114511006313
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F (2010) Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 13(5):654–662. https://doi.org/10.1017/s1368980009991698
Esfahani FH, Asghari G, Mirmiran P, Azizi F (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 20(2):150–158. https://doi.org/10.2188/jea.je20090083
Food composition table (FCT). Food and Nutrition Information Center, US Department of Agriculture 2010 [20 September 2009]. Available from: http://www.nal.usda.gov/fnic/foodcomp
Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr (1999) Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med 341(15):1097–1105
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/bf00280883
Badings H, De Jong C (1983) Glass capillary gas chromatography of fatty acid methyl esters. A study of conditions for the quantitative analysis of short-and long-chain fatty acids in lipids. J Chromatogr A 279:493–506
Rio DC, Ares M, Hannon GJ (2010) Nilsen TW (2010) Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protoc 6:pdb.prot5439
Desjardins P, Conklin D (2010) NanoDrop microvolume quantitation of nucleic acids. J Vis Exp JoVE. https://doi.org/10.3791/2565
Zhang WX, Fan J, Ma J, Rao YS, Zhang L, Yan YE (2016) Selection of suitable reference genes for quantitative real-time PCR normalization in three types of rat adipose tissue. Int J Mol Sci. https://doi.org/10.3390/ijms17060968
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Hulley S, Cummings S, Browner W, Grady D, Newman T (2013) Designing clinical research, vol 4. LWW, Philadelphia
Schober P, Boer C, Schwarte LA (2018) Correlation coefficients: appropriate use and interpretation. Anesth Analg 126(5):1763–1768
Nowacka-Woszuk J, Pruszynska-Oszmalek E, Szydlowski M, Szczerbal I (2017) Nutrition modulates Fto and Irx3 gene transcript levels, but does not alter their DNA methylation profiles in rat white adipose tissues. Gene 610:44–48. https://doi.org/10.1016/j.gene.2017.02.002
Zhong T, Duan XY, Zhang H, Li L, Zhang HP, Niu L (2017) Angelica sinensis suppresses body weight gain and alters expression of the FTO gene in high-fat-diet induced obese mice. Biomed Res Int 2017:6280972. https://doi.org/10.1155/2017/6280972
Perfilyev A, Dahlman I, Gillberg L, Rosqvist F, Iggman D, Volkov P, Nilsson E, Risérus U, Ling C (2017) Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am J Clin Nutr 105(4):991–1000
Muhlhausler B, Cook-Johnson R, James M, Miljkovic D, Duthoit E, Gibson R (2010) Opposing effects of omega-3 and omega-6 long chain polyunsaturated fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J Nutr Metab. https://doi.org/10.1155/2010/927836
Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR (2003) Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38(4):391–398
Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Vecka M, Tvrzicka E, Bryhn M, Kopecky J (2004) Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 39(12):1177–1185
Clarke SD (2000) Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr 83(S1):S59–S66
Terra X, Auguet T, Porras JA, Quintero Y, Aguilar C, Luna AM, Hernández M, Sabench F, del Castillo D, Richart C (2010) Anti-inflammatory profile of FTO gene expression in adipose tissues from morbidly obese women. Cell Physiol Biochem 26(6):1041–1050
Lappalainen T, Lindström J, Paananen J, Eriksson JG, Karhunen L, Tuomilehto J, Uusitupa M (2012) Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr 108(10):1859–1865
Steemburgo T, Azevedo MJ, Gross JL, Milagro FI, Campión J, Martínez JA (2013) The rs9939609 polymorphism in the FTO gene is associated with fat and fiber intakes in patients with type 2 diabetes. Lifestyle Genomics 6(2):97–106
Koochakpour G, Esfandiar Z, Hosseini-Esfahani F, Mirmiran P, Daneshpour M, Sedaghati-Khayat B, Azizi F (2019) Evaluating the interaction of common FTO genetic variants, added sugar, and trans-fatty acid intakes in altering obesity phenotypes. Nutr Metab Cardiovasc Dis 29(5):474–480
Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, Lai CQ, Lee YC, Warodomwichit D, Hopkins PN, Ordovas JM (2011) A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr 141(12):2219–2225. https://doi.org/10.3945/jn.111.143826
Yuzbashian E, Asghari G, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A (2019b) The association of dietary carbohydrate with FTO gene expression in visceral and subcutaneous adipose tissue of adults without diabetes. Nutrition 63:92–97
McMurray F, Church CD, Larder R, Nicholson G, Wells S, Teboul L, Tung YC, Rimmington D, Bosch F, Jimenez V, Yeo GS, O’Rahilly S, Ashcroft FM, Coll AP, Cox RD (2013) Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genet 9(1):e1003166. https://doi.org/10.1371/journal.pgen.1003166
Cheung MK, Gulati P, O’Rahilly S, Yeo GS (2013) FTO expression is regulated by availability of essential amino acids. Int J Obes 37(5):744–747. https://doi.org/10.1038/ijo.2012.77
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165(3):535–550
Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS (2005) Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol 15(3):331–341. https://doi.org/10.1016/j.sbi.2005.05.006
Acknowledgements
We express our appreciation to the participants of the study for their enthusiastic support and to the staff of the involved hospitals for their valuable help.
Funding
This work was funded by a Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
Author information
Authors and Affiliations
Contributions
EY conceptualized and designed the study, gathered adipose tissue, performed RNA extraction and Real-Time qRT-PCR, analyzed and interpreted the data, prepared the manuscript and approved the final manuscript as submitted. GA entered data, drafted the initial manuscript, and approved the final manuscript as submitted. CBC critically revised the manuscript and approved the final manuscript as submitted. MS performed gas-chromatography for fatty acid concentration and approved the final manuscript as submitted. MH supervised the project, consulted lab protocol, and approved the final manuscript as submitted. MZ prepared the lab materials; cDNA synthesized, prepared the manuscript and approved the final manuscript as submitted. PM drafted the initial manuscript and approved the final manuscript as submitted. AK biopsied the patients during the abdominal surgery and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author hereby declares that there is no conflict of interest.
Ethical approval
Ethics approval was obtained from the ethics committee of the Research Institute for Endocrine Sciences (RIES) of the Shahid Beheshti University of Medical Sciences (NO: IR.SBMU.ENDOCRINE.REC.1396.483) and the study was conducted in accordance with the Declaration of Helsinki and RIES institutional guidelines. Written informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Yuzbashian, E., Asghari, G., Chan, C.B. et al. The association of dietary and plasma fatty acid composition with FTO gene expression in human visceral and subcutaneous adipose tissues. Eur J Nutr 60, 2485–2494 (2021). https://doi.org/10.1007/s00394-020-02422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02422-x