Abstract
Purpose
Many studies have examined the association of isoflavone intake with type 2 diabetes (T2D), and produced inconsistent results. Few studies, however, explored the association using objective biomarkers (particular for daidzein metabolite-equol) of isoflavones. We aimed to explore the association of urinary equol, daidzein and genistein concentrations with T2D and examine the mediating roles of high-sensitivity C-reactive protein (hsCRP) and retinol binding protein 4 (RBP4).
Methods
This prospective study included 2818 subjects. Urinary concentrations of equol, daidzein and genistein were measured by high-performance liquid chromatography-tandem mass spectrometry. The associations between urinary isoflavones and T2D incidence were evaluated by cox proportional hazards model.
Results
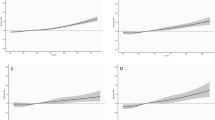
After adjustment for covariates, urinary equol except daidzein and genistein was inversely associated with T2D incidence. In comparison with the first tertile, multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for T2D incidence in the second and third tertile of equol concentration were 0.52 (0.37, 0.73) and 0.72 (0.53, 0.97), respectively. In stratified analyses by sex, the HR (95% CI) of men in the second vs. first tertile of equol was 0.29 (0.14, 0.58). Equivalent estimation in women was 0.67 (0.45, 1.01). Neither women nor men in the third tertile showed significant difference of T2D incidence compared with the first tertile. In path analyses, there was no evidence of mediating effects of hsCRP and RBP4 on the “equol-T2D” relationship.
Conclusions
Urinary equol was favorably associated with a decreased T2D incidence in Chinese adults. The equol–T2D relationship might not be mediated by hsCRP and RBP4.
Trial registration
This study has been registered at http://www.clinicaltrials.gov as NCT03179657.


Similar content being viewed by others
Abbreviations
- AGFI:
-
Adjusted goodness of fit index
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- ER:
-
Estrogen receptors
- FBG:
-
Fasting blood glucose
- FFQ:
-
Food-frequency questionnaire
- GFI:
-
The goodness of fit index
- GNHS:
-
The Guangzhou Nutrition and Health Study
- hsCRP:
-
High-sensitivity C-reactive protein
- HR:
-
Hazard ratio
- OR:
-
Odd ratio
- RBP4:
-
Retinol binding protein 4
- RCT:
-
Randomized controlled trial
- T:
-
Tertile
- T2D:
-
Type 2 diabetes
References
International Diabetes Federation (2017) IDF diabetes atlas, 8th edn. Brussels, Belgium
National Diabetes Research Group (1981) Diabetes mellitus survey of 300,000 in fourteen provinces and cities of China. Chin Med J 20:678–681
Setchell KD (1998) Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68:1333S–1346S. https://doi.org/10.1093/ajcn/68.6.1333S
Bhathena SJ, Velasquez MT (2002) Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 76:1191–1201. https://doi.org/10.1093/ajcn/76.6.1191
Vinayagam R, Xu B (2015) Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond) 12:60. https://doi.org/10.1186/s12986-015-0057-7
Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S (1995) Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr 125:2307–2315. https://doi.org/10.1093/jn/125.9.2307
Setchell KD, Clerici C (2010) Equol: history, chemistry, and formation. J Nutr 140:1355S–1362S. https://doi.org/10.3945/jn.109.119776
Setchell KD, Clerici C (2010) Equol: pharmacokinetics and biological actions. J Nutr 140:1363S–1368S. https://doi.org/10.3945/jn.109.119784
Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO (2006) Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol 101:246–253. https://doi.org/10.1016/j.jsbmb.2006.06.020
Villegas R, Gao Y-T, Yang G, Li H-L, Elasy TA, Zheng W, Shu XO (2008) Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr 87:162–716. https://doi.org/10.1093/ajcn/87.1.162
Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, Pereira MA (2012) Soy intake and risk of type 2 diabetes in Chinese Singaporeans. Eur J Nutr 51:1033–1040. https://doi.org/10.1007/s00394-011-0276-2
Ding M, Pan A, Manson J, Willett WC, Malik V, Rosner B, Giovannucci E, Hu FB, Sun Q (2016) Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur J Clin Nutr 70:1381–1387. https://doi.org/10.1038/ejcn.2016.117
Morimoto Y, Steinbrecher A, Kolonel LN, Maskarinec G (2011) Soy consumption is not protective against diabetes in Hawaii: the Multiethnic Cohort. Eur J Clin Nutr 65:279–282. https://doi.org/10.1038/ejcn.2010.228
Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA (2000) Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer 36:27–32. https://doi.org/10.1207/S15327914NC3601_5
Setchell KD, Brown NM, Lydeking-Olsen E (2002) The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 132:3577–3584. https://doi.org/10.1093/jn/132.12.3577
Setchell KD, Cole SJ (2006) Method of defining equol-producer status and its frequency among vegetarians. J Nutr 136:2188–2193. https://doi.org/10.1093/jn/136.8.2188
Ahuja V, Miura K, Vishnu A, Fujiyoshi A, Evans R, Zaid M, Miyagawa N, Hisamatsu T, Kadota A, Okamura T, Ueshima H, Sekikawa A (2017) Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men. Br J Nutr 117:260–266. https://doi.org/10.1017/S000711451600458X
Yoshikata R, Myint KZ, Ohta H (2017) Relationship between equol producer status and metabolic parameters in 743 Japanese women: equol producer status is associated with antiatherosclerotic conditions in women around menopause and early postmenopause. Menopause 24:216–224. https://doi.org/10.1097/GME.0000000000000743
Rienks J, Barbaresko J, Nothlings U (2017) Association of isoflavone biomarkers with risk of chronic disease and mortality: a systematic review and meta-analysis of observational studies. Nutr Rev 75:616–641. https://doi.org/10.1093/nutrit/nux021
Talaei M, Lee BL, Ong CN, van Dam RM, Yuan JM, Koh WP, Pan A (2016) Urine phyto-oestrogen metabolites are not significantly associated with risk of type 2 diabetes: the Singapore Chinese health study. Br J Nutr 115:1607–1615. https://doi.org/10.1017/S0007114516000581
Ko K-P, Kim C-S, Ahn Y, Park S-J, Kim Y-J, Park JK, Lim YK, Yoo KY, Kim SS (2015) Plasma isoflavone concentration is associated with decreased risk of type 2 diabetes in Korean women but not men: results from the Korean Genome and Epidemiology Study. Diabetologia 58:726–735. https://doi.org/10.1007/s00125-014-3463-x
Ho SC, Chen YM, Ho SS, Woo JL (2007) Soy isoflavone supplementation and fasting serum glucose and lipid profile among postmenopausal Chinese women: a double-blind, randomized, placebo-controlled trial. Menopause 14:905–912. https://doi.org/10.1097/GME.0b013e318032b2d3
Bakhtiary A, Yassin Z, Hanachi P, Rahmat A, Ahmad Z, Halalkhor S, Firouzjahi AR (2011) Evaluation of the oxidative stress and glycemic control status in response to soy in older women with the metabolic syndrome. Iran Red Crescent Med 13:795
Liu ZM, Chen YM, Ho SC, Ho YP, Woo J (2010) Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr 91:1394–1401. https://doi.org/10.3945/ajcn.2009.28813
Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S (2009) Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 16:395–400. https://doi.org/10.1097/gme.0b013e3181857979
Nicastro HL, Mondul AM, Rohrmann S, Platz EA (2013) Associations between urinary soy isoflavonoids and two inflammatory markers in adults in the United States in 2005–2008. Cancer Causes Control 24:1185–1196. https://doi.org/10.1007/s10552-013-0198-9
Wu SH, Shu XO, Chow WH, Xiang YB, Zhang X, Li HL, Cai Q, Ji BT, Cai H, Rothman N, Gao YT, Zheng W, Yang G (2012) Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet 112:996–1004. https://doi.org/10.1016/j.jand.2012.04.001
Liu J, Shi WQ, Cao Y, He LP, Guan K, Ling WH, Chen YM (2014) Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br J Nutr 112:2041–2048. https://doi.org/10.1017/S000711451400316X
Zeng FF, Wu BH, Fan F, Xie HL, Xue WQ, Zhu HL, Chen YM (2013) Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab 98:2347–2355. https://doi.org/10.1210/jc.2013-1190
Cao WT, Zeng FF, Li BL, Lin JS, Liang YY, Chen YM (2018) Higher dietary carotenoid intake associated with lower risk of hip fracture in middle-aged and elderly Chinese: a matched case-control study. Bone 111:116–122. https://doi.org/10.1016/j.bone.2018.03.023
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS (2011) 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43:1575–1581. https://doi.org/10.1249/MSS.0b013e31821ece12
Yang YX, Wang GY, Pan XC (2009) China Food Composition Table 2009. Peking University, Beijing
Wang L, Lu WH, ChEng C,L, Xie FQ, Wei HL, Cui HB (2009) The simultaneous analysis of six isoflavone metabolites in human urine by HPLC. Acta Nutr Sin 31:181–184
He J, Feng Y, Ouyang HZ, Yu B, Chang YX, Pan GX, Dong GY, Wang T, Gao XM (2013) A sensitive LC–MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves. J Pharm Biomed Anal 84:189–195. https://doi.org/10.1016/j.jpba.2013.06.019
American Diabetes Association (2014) Standards of medical care in diabetes—2014. Diabetes Care 37:S14–S80. https://doi.org/10.2337/dc14-S014
Jackman KA, Woodman OL, Sobey CG (2007) Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr Med Chem 14:2824–2830. https://doi.org/10.2174/092986707782360178
Jackson RL, Greiwe JS, Schwen RJ (2011) Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr Rev 69:432–448. https://doi.org/10.1111/j.1753-4887.2011.00400.x
Shi L, Ryan HH, Jones E, Simas TA, Lichtenstein AH, Sun Q, Hayman LL (2014) Urinary isoflavone concentrations are inversely associated with cardiometabolic risk markers in pregnant U.S. women. J Nutr 144:344–351. https://doi.org/10.3945/jn.113.184069
Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, Shimatsu A, Inaguma A, Ueno T, Uchiyama S, Satoh-Asahara N (2013) Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol (Oxf) 78:365–372. https://doi.org/10.1111/j.1365-2265.2012.04400.x
Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A (2014) Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr 100:11–26. https://doi.org/10.3945/ajcn.113.077743
Grosso G, Stepaniak U, Micek A, Kozela M, Stefler D, Bobak M, Pajak A (2017) Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br J Nutr 118:60–68. https://doi.org/10.1017/S0007114517001805
Tresserra-Rimbau A, Guasch-Ferre M, Salas-Salvado J et al (2016) Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J Nutr pii:jn223610. https://doi.org/10.3945/jn.115.223610
Perez-Jimenez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A (2010) Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 92:801–809. https://doi.org/10.3945/ajcn.2010.29924
Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB, Sun Q (2015) Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr 114:1694–1701. https://doi.org/10.1017/S0007114515003359
Zhang YB, Chen WH, Guo JJ, Fu ZH, Yi C, Zhang M, Na XL (2013) Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women–a meta-analysis. Nutrition 29:8–14. https://doi.org/10.1016/j.nut.2012.03.019
Ye YB, Chen AL, Lu W, Zhuo SY, Liu J, Guan JH, Deng WP, Fang S, Li YB, Chen YM (2015) Daidzein and genistein fail to improve glycemic control and insulin sensitivity in Chinese women with impaired glucose regulation: a double-blind, randomized, placebo-controlled trial. Mol Nutr Food Res 59:240–249. https://doi.org/10.1002/mnfr.201400390
Cooke PS, Naaz A (2004) Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 229:1127–1135
Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M (2011) The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol 650:694–702. https://doi.org/10.1016/j.ejphar.2010.10.060
Blay M, Espinel AE, Delgado MA, Baiges I, Blade C, Arola L, Salvado J (2010) Isoflavone effect on gene expression profile and biomarkers of inflammation. J Pharm Biomed Anal 51:382–390. https://doi.org/10.1016/j.jpba.2009.03.028
Choi MS, Jung UJ, Yeo J, Kim MJ, Lee MK (2008) Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab Res Rev 24:74–81. https://doi.org/10.1002/dmrr.780
Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N (2003) Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr 133:1238–1243. https://doi.org/10.1093/jn/133.5.1238
Manach C, Williamson G, Morand C, Scalbert A, Remesy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S. https://doi.org/10.1093/ajcn/81.1.230S
Franke AA, Hebshi SM, Pagano I, Kono N, Mack WJ, Hodis HN (2010) Urine accurately reflects circulating isoflavonoids and ascertains compliance during soy intervention. Cancer Epidemiol Biomarkers Prev 19:1775–1783. https://doi.org/10.1158/1055-9965
Lee SA, Wen W, Xiang YB, Barnes S, Liu D, Cai Q, Zheng W, Shu XO (2007) Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr 137:1011–1016. https://doi.org/10.1093/jn/137.4.1011
Acknowledgements
We thank other participants and staff who contributed to the present study.
Funding
This study was jointly supported by the National Natural Science Foundation of China (Nos. 81472965 and 81502798), Natural Science Foundation of Guangdong Province, China (No. 2015A030310399), and the 5010 Program for Clinical Researches (No. 2007032) of the Sun Yat-sen University (Guangzhou, China).
Author information
Authors and Affiliations
Contributions
YMC designed the research. HLD, XYT, YYD, QWZ and CW conducted research. HLD analyzed the data. HLD wrote the paper. YMC and ZQZ critically revised the manuscript. YMC had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, HL., Tang, XY., Deng, YY. et al. Urinary equol, but not daidzein and genistein, was inversely associated with the risk of type 2 diabetes in Chinese adults. Eur J Nutr 59, 719–728 (2020). https://doi.org/10.1007/s00394-019-01939-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01939-0