Abstract
Purpose
Barley is a low-glycemic index grain that can help diabetic and obese patients. The effect of barley intake depends on the host and the associated gut microbiota. This study investigated the effect of barley intake on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation.
Methods
Obese db/db mice were fed diets with and without barley during 8 weeks; lean mice were used as lean controls. Fecal microbiota was evaluated using 16S marker gene sequencing in a MiSeq instrument; several markers of caecal biochemistry, obesity, and inflammation were also evaluated using standard techniques.
Results
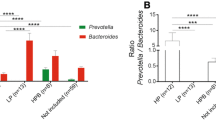
Bacterial richness (i.e., Operational Taxonomic Units) and Shannon diversity indexes were similar in all obese mice (with and without barley) and higher compared to lean controls. Barley intake was associated with increased abundances of Prevotella, Lactobacillus, and the fiber-degraders S24-7 (Candidatus Homeothermaceae) compared to both lean and obese controls. The analysis of unweighted UniFrac distances showed a separate clustering of samples for each experimental group, suggesting that consumption of barley contributed to a phylogenetically unique microbiota distinct from both obese and lean controls. Caecal butyrate concentrations were similar in all obese mice, while succinic acid was lower in the barley group compared to obese controls. Barley intake was also associated with lower plasma insulin and resistin levels compared to obese controls.
Conclusions
This study shows that barley intake is associated with a different fecal microbiota, caecal biochemistry, and obesity biomarkers in db/db mice that tend to be more similar to lean controls.






Similar content being viewed by others
References
Shapira M (2016) Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol Evol 31(7):539–549. doi:10.1016/j.tree.2016.03.006
Clemente JC, Ursell LK, Wegener Parfrey L, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270. doi:10.1016/j.cell.2012.01.035
Rangel-Huerta OD, Aguilera CM, Martin MV, Soto MJ, Rico MC, Vallejo F et al (2015) Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr 145(8):1808–1816. doi:10.3945/jn.115.213660
Carson TL, Hidalgo B, Ard JD, Affuso O (2014) Dietary interventions and quality of life: a systematic review of the literature. J Nutr Educ Behav 46(2):90–101. doi:10.1016/j.jneb.2013.09.005
Janssen AW, Kersten S (2015) The role of the gut microbiota in metabolic health. FASEB J 29(8):3111–3123. doi:10.1096/fj.14-269514
NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurements studies with 19.2 million participants. Lancet 387:1377–1396. doi:10.1016/S0140-6736(16)30054-X
Ojeda P, Bobe A, Dolan K, Leone V, Martinez K (2016) Nutritional modulation of gut microbiota—the impact on metabolic disease pathophysiology. J Nutr Biochem 28:191–200. doi:10.1016/j.jnutbio.2015.08.013
Noratto GD, Garcia-Mazcorro JF, Markel M, Martino HS, Minamoto Y, Steiner JM et al (2014) Carbohydrate-free peach (Prunus persica) and plum (Prunus salicina) juice affects fecal microbial ecology in an obese animal model. PLoS One 9(7):e101723. doi:10.1371/journal.pone.0101723
Howe A, Ringus DL, Williams RJ, Choo Z-N, Greenwald SM, Owens SM et al (2015) Divergent responses of viral and bacterial communities in the gut microbiome to dietary disturbances in mice. ISMEJ 10:1217–1227. doi:10.1038/ismej.2015.183
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12(1):5–9. doi:10.1038/ni0111-5
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108. doi:10.1126/science.1208344
Baik BK, Ullrich SE (2008) Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci 48:233–242. doi:10.1016/j.jcs.2008.02.002
Wilson TA, Nicolosi RJ, Delaney B, Chadwell K, Moolchandani V, Kotyla T et al (2004) Reduced and high molecular weight barley β-glucans decrease plasma total and non-HDL cholesterol in hypercholesterolemic Syrian golden hamsters. J Nutr 134(10):2617–2622
Pins JJ, Kaur H, Dodds E, Keenan JM (2007) The effects of cereal fibers and barley foods rich in beta-glucan on cardiovascular disease and diabetes risk. In: Marquart L, Jacobs DR Jr, McIntosh GH, Poutanen K, Reicks M (eds) Whole grains and health. Blckwell, London, pp 75–85
DeAngelis M, Montemurno E, Vannini L, Cosola C, Cavallo N, Gozzi G et al (2015) Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl Environ Microbiol 81:7945–7956. doi:10.1128/AEM.02507-15
Philippeau C, Sadet-Bourgeteau S, Varloud M, Julliand V (2015) Impact of barley form on equine total tract fibre digestibility and colonic microbiota. Animal 9:1943–1948. doi:10.1017/S1751731115001524
Wang B, Chandrasekera PC, Pippin JJ (2014) Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10(2):131–145. doi:10.2174/1573399810666140508121012
Jonker D, Hasselwander O, Tervilä-Wilo A, Tenning PP (2010) 28-Day oral toxicity study in rats with high purity barley beta-glucan (Glucagel). Food Chem Toxicol 48(1):422–428. doi:10.1016/j.fct.2009.10.034
Jeyakumar SM, Vajreswari A, Giridharan NV (2006) Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity 14(1):52–59. doi:10.1038/oby.2006.7
Garcia-Mazcorro JF, Ivanov I, Mills DA, Noratto G (2016) Influence of whole-wheat consumption on fecal microbial structure of obese diabetic mice. PeerJ 4:e1702. doi:10.7717/peerj.1702
Garcia-Mazcorro JF, Mills D, Noratto G (2016) Molecular exploration of fecal microbiome in quinoa-supplemented obese mice. FEMS Microbiol Ecol 92(7):fiw089. doi:10.1093/femsec/fiw089
Bokulich NA, Thorngate JH, Richardson PM, Mills DA (2014) Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci USA 111(1):E139–E148. doi:10.1073/pnas.1317377110
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al (2009) Introducing mothur: open source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Envion Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi:10.1038/nmeth.f.303
Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu Z et al (2013) Advancing our understanding of the human microbiome using QIIME. Methods Enzymol 531:371–444. doi:10.1016/B978-0-12-407863-5.00019-8
Rideout JR, He Y, Navas-Molina JA, Walters WA, Ursell LK, Gibbons SM et al (2014) Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ 2:e545. doi:10.7717/peerj.545
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi:10.1093/bioinformatics/btq461
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi:10.1128/AEM.03006-05
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi:10.1038/nbt.2676
Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M (2012) KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:D109–D114. doi:10.1093/nar/gkr988
Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, Pedreschi R (2012) Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem 135:1592–1599. doi:10.1016/j.foodchem.2012.05.088
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Hawkins CL, Morgan PE, Davies MJ (2009) Quantification of protein modification by oxidants. Free Radic Biol Med 46:965–988. doi:10.1016/j.freeradbiomed.2009.01.007
Alam MA, Sernia C, Brown L (2013) Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J Cardiovasc Pharmacol 61:240–249. doi:10.1097/FJC.0b013e31827cb600
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:1–9
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi:10.1128/AEM.71.12.8228-8235.2005
Lozupone CA, Hamady M, Kelley ST, Knight R (2007) Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 73:1576–1585. doi:10.1128/AEM.01996-06
Segata N, Izard J, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi:10.1186/gb-2011-12-6-r60
Parks DH, Beiko RG (2010) Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26:715–721. doi:10.1093/bioinformatics/btq041
Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243
Corrêa-Oliveira R, Fachi JL, Vieria A, Sato FT, Vinolo MA (2016) Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5:e73. doi:10.1038/cti.2016.17
Egger G, Dixon J (2009) Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics? Obes Rev 10(2):237–249. doi:10.1111/j.1467-789X.2008.00542.x
Fresno M, Alvarez R, Cuesta N (2011) Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem 117(3):151–164. doi:10.3109/13813455.2011.562514
Venkatesan V, Madhira SL, Malakapalli VM, Chalasani M, Shaik SN, Seshadri V et al (2013) Obesity, insulin resistance, and metabolic syndrome: a study in WNIN/Ob rats from a pancreatic perspective. Biomed Res Int. doi:10.1155/2013/617569
Triantafyllou GA, Paschou SA, Mantzoros CS (2016) Leptin and hormones: energy homeostasis. Endocrinol Metab Clin N Am 45:633–645. doi:10.1016/j.ecl.2016.04.012
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM et al (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312. doi:10.1038/35053000
Nilsson AC, Johansson-Boll EV, Björck IME (2015) Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross-over study in healthy middle-aged subjects. Br J Nutr 114(06):899. doi:10.1017/S0007114515002524
Anderson JW, Bridges SR (1993) Hypocholesterolemic effects of oat bran in humans. In: Wood PJ (ed) oat bran. American Association of Cereal Chemists, St. Paul, pp 139–157
Tong LT, Zhong K, Liu L, Zhou X, Qiu J, Zhou S (2015) Effects of dietary hull-less barley β-glucan in the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem 169:344–349. doi:10.1016/j.foodchem.2014.07.157
Wursch P, Pi-Sunyer FX (1997) The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereal rich in beta-glucan. Diabetes Care 20:1774–1780
Bluher M (2016) Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci 130:1603–1614. doi:10.1042/CS20160005
USDA Food Composition Databases. National Nutrient Database for Standard Reference Release 28, released September 2015, slightly revised May 2016. https://ndb.nal.usda.gov/ndb/ (retrieved on Nov 2016)
McRorie JW, McKeown NM (2017) Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet 117(2):251–264. doi:10.1016/j.jand.2016.09.021
Belobrajdic DP, Jobling SA, Morell MK, Taketa S, Bird AR (2015) Wholegrain barley beta-glucan fermentation does not improve glucose tolerance in rats fed a high-fat diet. Nutr Res 35(2):162–168. doi:10.1016/j.nutres.2014.12.006
Zhong Y, Nyman M, Fåk F (2015) Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res 59:2066–2076. doi:10.1002/mnfr.201500187
Wang Y, Ames NP, Tun HM, Tosh SM, Jones PJ, Khafipour E (2016) High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol 7:129. doi:10.3389/fmicb.2016.00129
Moen B, Berget I, Rud I, Hole AS, Kjos NP, Sahlstrøm S (2016) Extrusion of barley and oat influence the fecal microbiota and SCFA profile of growing pigs. Food Funct 7:1024–1032. doi:10.1039/c5fo01452b
Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C et al (2012) Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61:543–553. doi:10.1136/gutjnl-2011-301012
Shen TC, Chehoud C, Ni J, Hsu E, Chen YY, Bailey A et al (2016) Dietary regulation of the gut microbiota engineered by a minimal defined bacterial consortium. PLoS One 11:e0155620. doi:10.1371/journal.pone.0155620
Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD et al (2016) Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 4:36. doi:10.1186/s40168-016-0181-2
Hughes SA, Shewry PR, Gibson GR, McCleary BV, Rastall RA (2008) In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol Ecol 64:482–493. doi:10.1111/j.1574-6941.2008.00478.x
Kovatcheva-Datchary P, Egert M, Maathuis A, Rajilić-Stojanović M, de Graaf AA, Smidt H et al (2009) Linking phylogenetic identities of bacteria to starch fermentation in an in vitro model of the large intestine by RNA-based stable isotope probing. Environ Microbiol 11:914–926. doi:10.1111/j.1462-2920.2008.01815.x
Hoyles L, McCartney AL (2009) What do we mean when we refer to Bacteroidetes populations in the human gastrointestinal microbiota? FEMS Microbiol Lett 299:175–183. doi:10.1111/j.1574-6968.2009.01741.x
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2015) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102:11070–11075. doi:10.1073/pnas.0504978102
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P et al (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 32:1720–1724. doi:10.1038/ijo.2008.155
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 106(7):2365–2370. doi:10.1073/pnas.0812600106
Oki K, Toyama M, Banno T, Chonan O, Benno Y, Watanabe K (2016) Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol 16:284. doi:10.1186/s12866-016-0898-x
Ziętak M, Kovatcheva-Datchary P, Markiewicz LH, Ståhlman M, Kozak LP, Bäckhed F (2016) Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab 23:1216–1223. doi:10.1016/j.cmet.2016.05.001
Baldwin J, Collins B, Wolf PG, Martinez K, Shen W, Chuang CC et al (2016) Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J Nutr Biochem 27:123–135. doi:10.1016/j.jnutbio.2015.08.027
Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN et al (2016) Cospeciation of gut microbiota with hominids. Science 353:380–382. doi:10.1126/science.aaf3951
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human microbiota. Nature 489:220–230. doi:10.1038/nature11550
Weiss E, Aumiller T, Spindler HK, Rosenfelder P, Eklund M, Witzig M et al (2016) Wheat and barley differently affect porcine intestinal microbiota. J Sci Food Agric 96:2230–2239. doi:10.1002/jsfa.7340
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M et al (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517. doi:10.2337/db08-1637
Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF (2016) The neuropharmacology of butyrate: the bread and butter of the microbiota–gut–brain axis? Neurochem Int 99:110–132. doi:10.1016/j.neuint.2016.06.011
Inagaki A, Ichikawa H, Sakata T (2007) Inhibitory effect of succinic acid on epithelial cell proliferation of colonic mucosa in rats. J Nutr Sci Vitaminol (Tokyo) 53:377–379. doi:10.3177/jnsv.53.377
Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M (2013) High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, live fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One 8:e80476. doi:10.1371/journal.pone.0080476
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8. doi:10.1111/j.1574-6968.2009.01514.x
Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R et al (2015) Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6:164. doi:10.3389/fphys.2015.00164
Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL (2014) Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 16(6):770–777. doi:10.1016/j.chom.2014.11.003
Brockman DA, Chen X, Gallaher DD (2013) Consumption of a high beta-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr 52:1743–1753. doi:10.1007/s00394-012-0478-2
Zhong Y, Marungruang N, Fak F, Nyman M (2015) Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br J Nutr 113:1558–1570. doi:10.1017/S0007114515000793
Pfalzer AC, Nesbeth PDC, Parnell LD, Iyer LK, Liu Z, Kane AV et al (2015) Diet- and genetically-induced obesity differentially affect the fecal microbiome and metabolome in Apc1638N mice. PLoS One 10:e0135758. doi:10.1371/journal.pone.0135758
Park HK, Kwak MK, Kim HJ, Ahima RS (2017) Linking resistin, inflammation, and cardiometabolic diseases. Koren J Intern Med 32(2):239–247. doi:10.3904/kjim.2016.229
Fujimoto S, Mochizuki K, Goda T (2010) Gene expression of inflammatory cytokines in peripheral leukocytes in db/db mice rose with progression of diabetes. Biosci Biotechnol Biochem 74(7):1488–1490. doi:10.1271/bbb.100149
Ladefoged M, Buschard K, Hansen AM (2013) Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS 121(6):531–538. doi:10.1111/apm.12018
Seto SW, Lam TY, Or PM, Lee WY, Au AL, Poon CC et al (2010) Folic acid consumption reduces resistin level and restores blunted acetylcholine-induced aortic relaxation in obese/diabetic mice. J Nutr Biochem 21(9):872–880. doi:10.1016/j.jnutbio.2009.06.015
Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A et al (2005) Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia 48(5):984–992. doi:10.1007/s00125-005-1735-1
Saint-Georgees-Chaumet Y, Edeas M (2016) Microbiota–mitochondria inter-talk: consequence for microbiota–host interaction. Pathog Dis 74(1):ftv096. doi:10.1093/femspd/ftv096
Ames NP, Rhymer CR (2008) Issues surrounding health claims for barley. J Nutr 138:1237S–1243S
Lang JM, Eisen JA, Zivkovic AM (2014) The microbes we eat: abundance and taxonomy of microbes consumed in a day’s worth for three diet types. PeerJ 2:3659. doi:10.7717/peerj.659
Blancas-Velazquez A, Mendoza J, Garcia AN, la Fleur SE (2017) Diet-induced obesity and circadian disruption of feeding behavior. Front Neurosci 11:23. doi:10.3389/fnins.2017.00023
Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23(2):270–299. doi:10.1017/S0954422410000168
Mercer JG, Archer ZA (2008) Putting the diet back into diet-induced obesity: diet-induced hypothalamic gene expression. Eur J Pharmacol 585(1):31–37. doi:10.1016/j.ejphar.2007.11.077
Mobbs CV, Mastaitis J, Yen K, Schwartz J, Mohan V, Poplawski M et al (2007) Low-carbohydrate diets cause obesity, low-carbohydrate diets reverse obesity: a metabolic mechanism resolving the paradox. Appetite 48(2):135–138. doi:10.1016/j.appet.2006.06.007
Ding C, Guo J, Su Z (2015) The status of research into resistance to diet-induced obesity. Horm Metab Res 47(6):404–410. doi:10.1055/s-0034-1395584
Acknowledgements
DAM acknowledges the Peter J. Shields Endowed Chair. The authors would like to express their deepest gratitude to the QIIME and PICRUSt Help Forums for all the support provided. The authors would also like to thank Alejandra Mencia for her technical assistance in the analysis of blood parameters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garcia-Mazcorro, J.F., Mills, D.A., Murphy, K. et al. Effect of barley supplementation on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation in obese db/db mice. Eur J Nutr 57, 2513–2528 (2018). https://doi.org/10.1007/s00394-017-1523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1523-y