Abstract
Background
Type 2 diabetes (T2D), one of the major common human health problems, is growing at an alarming rate around the globe. Alpha-glucosidase and dipeptidyl peptidase IV (DPP-IV) enzymes play a significant role in development of T2D. Hence, reduction or inhibition of their activity can be one of the important strategies in management of T2D. Studies in the field of bioactive peptides have shown that dietary proteins could be natural source of alpha-glucosidase and DPP-IV inhibitory peptides.
Purpose
The purpose of this review is to provide an overview of food protein-derived peptides as potential inhibitors of alpha-glucosidase and DPP-IV with major focus on milk proteins.
Methods
Efforts have been made to review the available information in literature on the relationship between food protein-derived peptides and T2D. This review summarizes the current data on alpha-glucosidase and dipeptidyl peptidase IV inhibitory bioactive peptides derived from proteins and examines the potential value of these peptides in the treatment and prevention of T2D. In addition, the proposed modes of inhibition of peptide inhibitors are also discussed.
Results
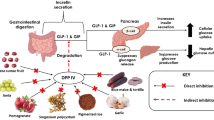
Studies revealed that milk and other food proteins-derived bioactive peptides play a vital role in controlling T2D through several mechanisms, such as the satiety response, regulation of incretin hormones, insulinemia levels, and reducing the activity of carbohydrate degrading digestive enzymes.
Conclusions
The bioactive peptides could be used in prevention and management of T2D through functional foods or nutraceutical supplements. Further clinical trials are necessary to validate the findings of in vitro studies and to confirm the efficiency of these peptides for applications.





Similar content being viewed by others
References
IDF (2013) IDF diabetes atlas, 6th edn. International Diabetes Federation, Brussels
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
UK Prospective Diabetes Study (UKPDS) Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317:703–713
Tiwari A, Rao JM (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 83:30–38
Avignon A, Radauceanu A, Monnier L (1997) Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 20:1822–1826
Abrahaamson MJ (2004) Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med 164:486–491
Jermendy G (2005) Can type 2 diabetes mellitus be considered preventable? Diabetes Res Clin Pract 68:S73–S81
Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D (2009) Dietary strategies for patients with type 2 diabetes in the era of multi-approaches review and results from the dietary intervention randomized controlled trial (DIRECT). Diabetes Res Clin Pract 86:S41–S48
Bantle Jp, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ (2008) Nutrition recommendations and interventions for diabetes: a position statement of the American diabetes association. Diabetes Care 31:S61–S78
Hawley JA, Gibala MJ (2012) What’s new since Hippocrates? Preventing type 2 diabetes by physical exercise and diet. Diabetologia 55:535–539
Holman RR, Cull CA, Turner RC (1999) A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UK Prospective Diabetes Study 44). Diabetes Care 22:960–964
Toeller M (1994) α-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest 24:31–35
Clissold SP, Edwards C (1988) A preliminary review of its pharmacodynamic and pharmacokinetics properties, and therapeutic potential. Drugs 35:214–243
Saito N, Sakai H, Sekihara H, Yajima Y (1998) Effect of an α-glucosidase inhibitor (voglibose) in combination with sulphonilureas, on glycemic control in type 2 diabetes patients. J Int Med Res 26:219–232
Bennett WL, Wilson LM, Bolen S et al (2011) Oral diabetes medications for adults with type 2 diabetes: an update. Comparative Effectiveness Rev. 27. http://www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed 09 Nov 2014
Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future development. Lancet 378:182–197
American Diabetes Association (2015) Standards of medical care in diabetes-2015. Diabetes Care 38:S1–S98
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35:1364–1379
Agius R (2014) An update on pharmacotherapy for type 2 diabetes. Malta Med J 26:29–38
Evert AB, Boucher JL, Cypress M et al (2014) Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 37(Suppl 1):S120–S143
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 39:157–163
Kitts DD, Weiler K (2003) Bioactive proteins and peptide from food sources: application of bioprocesses used in isolation and recovery. Curr Pharm Des 9:1309–1323
Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16:945–960
Phelan M, Kerins D (2011) The potential role of milk-derived peptides in cardiovascular disease. Food Funct 2:153–167
Tidona F, Criscione A, Guastella AM, Zuccaro A, Bordonaro S, Marletta D (2009) Bioactive peptides in dairy products. Ital J Anim Sci 8:315–340
Korhonen H (2009) Milk-derived bioactive peptides: from science to applications. J Funct Foods 1:177–187
Seppo L, Jauhiainen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure lowering effect in hypertensive subjects. Am J Clin Nutr 77:326–330
Haug A, Hostmark AT, Harstad OM (2007) Bovine milk in human nutrition–a review. Lipids Health Dis 6:1–16
Ebringer L, Ferenčík M, Krajčovič J (2008) Beneficial health effects of milk and fermented dairy products-review. Folia Microbiol 53:378–394
Atanasova J, Ivanova I (2010) Antibacterial peptides from goat and sheep milk proteins. Biotechnol Biotechnol Equip 24:1799–1803
Meisel H (1998) Overview on milk protein-derived peptides. Int Dairy J 8:363–373
Ricci-Cabello I, Olalla Herrera M, Artacho R (2012) Possible role of milk derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr Rev 70:241–255
Lacroix IM, Li-Chan ECY (2013) Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J Agric Food Chem 61:7500–7506
Konrad B, Anna D, Marek S, Marta P, Aleksandra Z, Jozefa C (2014) The evaluation of dipeptidyl peptidase (DPP)-IV, α-Glucosidase and angiotensin converting enzyme (ACE) inhibitory activities of whey proteins hydrolyzed with serine protease isolated from asian pumpkin (Cucurbita ficifolia). Int J Pept Res Ther 20:483–491
Frid AH, Nilsson M, Holst JJ, Björck IME (2005) Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 82:69–75
Manders RJF, Wagenmakers AJM, Koopman R (2005) Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr 82:76–83
Korhonen H, Pihlanto A (2007) Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr Pharm Des 13:829–843
Jakubowicz D, Froy O (2013) Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J Nutr Biochem 24:1–5
Graf S, Egert S, Heer M (2011) Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care 14:569–580
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci 94:14930–14935
Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P (2001) The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280:E340–E348
Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IME (2004) Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 80:1246–1253
Nilsson M, Holst JJ, Björck IM (2007) Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 85:996–1004
Calbet JA, Holst JJ (2004) Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr 43:127–139
McGregor RA, Poppitt SD (2013) Milk protein for improved metabolic health:a review of the evidence. Nutr Metab 10:46
Pistrosch F, Natali A, Hanefeld M (2011) Is hyperglycemia a cardiovascular risk factor? Diabetes Care 34:S128–S131
Gerich JE (2003) Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 16:1306–1316
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH (2010) Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 91:966–975
Pal S, Ellis V (2010) The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr 104:1241–1248
Claessens M, Calame W, Siemensma AD, Van Baak MA, Saris WHM (2009) The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur J Clin Nutr 63:48–56
Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V (2009) A whey protein supplement decreases post-prandial glycemia. Nutr J 8:1475–2891
Drucker DJ (2006) Enhancing the action of incretin hormones: a new whey forward? Endocrinology 147:3171–3172
Manders RJF, Praet SFE, Meex RCR, Koopman R, de Roos AL, Wagenmakers AJM, Saris WHM, van Loon LJ (2006) Protein hydrolysate/leucine co-ingestion reduces the prevalence of hyperglycemia in type 2 diabetic patients. Diabetes Care 29:2721–2722
Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tomé D (2012) Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev 25:29–39
Jones KW, Eller LK, Parnell JA, Doyle-Baker PK, Edwards AL, Reimer RA (2013) Effect of a dairy and calcium rich diet on weight loss and appetite during energy restriction in overweight and obese adults: a randomized trial. Eur J Clin Nutr 67:371–376
Diepvens K, Häberer D, Westerterp-Plantenga M (2008) Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes 32:510–518
Lorenzen J, Frederiksen R, Hoppe C, Hvid R, Astrup A (2012) The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur J Clin Nutr 66:622–627
Hernández-Ledesma B, García-Nebot MJ, Fernández-Tomé S, Amigo L, Recio I (2013) Dairy protein hydrolysates: peptides for health benefits. Int Dairy J 38:82–100. doi:10.1016/j.idairyj.2013.11.004
Hall WL, Millward DJ, Long SJ, Morgan LM (2003) Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr 89:239–248
Kazafeos K (2011) Incretin effect: GLP-1, GIP, DPP4. Diabetes Res Clin Pract 93(Suppl 1):S32–S36
Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I (2012) The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab 9:48
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP (2009) Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 96:675–682
Nauck MA, Vilsboll T, Gallwitz B, Garber A, Madsbad S (2009) Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care 32(Suppl 2):S223–S231
Portha B, Tourrel-Cuzin C, Movassat J (2011) Activation of the GLP-1 receptor signaling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. doi:10.1155/2011/376509
Halton TL, Hu FB (2004) The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23:373–385
Anderson GH, Tecimer SN, Shah D, Zafar TA (2004) Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr 134:3011–3015
Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A (2005) Brain amino acid requirements and toxicity: the example of leucine. J Nutr 135:1531S–1538S
Morrison CD, Xi X, White CL, Ye J, Martin RJ (2007) Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab 293:E165–E171
Lacroix IME, Li-Chan ECY (2012) Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int Dairy J 25:97–102
Tulipano G, Sibilia V, Caroli AM, Cocchi D (2011) Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 32:835–838
Uenishi H, Kabuki T, Seto Y, Serizawa A, Nakajima H (2012) Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int Dairy J 22:24–30
Yao Y, Sang W, Zhou M, Ren G (2010) Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J Agric Food Chem 58:770–774
Jaiswal N, Srivastava SP, Bhatia V, Mishra A, Sonkar AK (2012) Inhibition of alpha-glucosidase by acacia nilotica prevents hyperglycemia along with improvement of diabetic complications via aldose reductase inhibition. J Diabetes Metab 6:1–7
Apostolidis E, Kwon YI, Ghaedian R, Shetty K (2007) Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol 21:217–236
Slama G, Elgrably F, Mbemba J, Larger E (2006) Postprandial glycaemia:a plea for the frequent use of delta postprandial glycaemia in the treatment of diabetic patients. Diabetes Metab 32:187–192
Lee A, Patrick P, Wishart J, Horowitz M, Morley JE (2002) The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 4:329–335
Yusuke Moritoh, Takeuchi Koji, Hazama Masatoshi (2009) Voglibose, an alpha-glucosidase inhibitor, to increase active glucagon-like peptide-1 levels. Mol Cell Pharmacol 1:188–192
Campbell LK, Baker DE, Campbell RK (2000) Miglitol: assessment of its role in the treatment of patients with diabetes mellitus. Ann Pharmacother 34:1291–1301
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents:current role in type 2 diabetes mellitus. Drugs 65:385–411
Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B (2006) Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 49:1711–1721
McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D (2005) Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J Agric Food Chem 53:2760–2766
da Silva Pinto M, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K (2008) Functionality of bioactive compounds in Brazilian strawberry (Fragaria ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem 56:4386–4392
McCue P, Kwon YI, Shetty K (2005) Anti-amylase, anti-glucosidase and anti-angiotensin I-converting enzyme potential of selected foods. J Food Biochem 29:278–294
Matsui T, Yoshimoto C, Osajima K, Oki T, Osajima Y (1996) In vitro survey of α-glucosidase inhibitory food components. Biosci Biotechnol Biochem 60:2019–2022
Yu Z, Yin Y, Zhao W, Yu Y, Liu B, Liu J, Chen F (2011) Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem 129:1376–1382
Aschenbrenner DS, Venable SJ (2009) Drug therapy in nurshing, 3rd edn. Wolters Kluwer Health, New York, pp 589–592
Ross SA, Gulve EA, Wang M (2004) Chemistry and biochemistry of type 2 diabetes. Chem Rev 104:1255–1282
Chiasson JL, Rabasa-Lhoret R (2004) Prevention of type 2 diabetes:insulin resistance and beta-cell function. Diabetes 53:S34–S38
Arungarinathan G, McKay GA, Fisher M (2011) Drugs for diabetes: part 4 acarbose. Br J Cardiol 18:78–81
Holst JJ (2004) On the physiology of GIP and GLP-1. Horm Metab Res 36:747–754
Gromada J, Rorsman P (2004) New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res 36:822–829
Perfetti R, Hui H (2004) The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res 36:804–810
Goto Y, Yamada K, Ohyama T, Matsuo T, Odaka H, Ikeda H (1995) An alpha-glucosidase inhibitor, AO-128, retards carbohydrate absorption in rats and humans. Diabetes Res Clin Pract 28:81–87
Gribble FM, Williams L, Simpson AK, Reimann F (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154
Tolhurst G, Reimann F, Gribble FM (2009) Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587:27–32
Moritoh Y, Takeuchi K, Hazama M (2009) Chronic administration of voglibose, an alpha-glucosidase inhibitor, increases active glucagon-like peptide-1 levels by increasing its secretion and decreasing dipeptidyl peptidase-F4 activity in ob/ob mice. J Pharmacol Exp Ther 329:669–676
Nauck MA (1998) Glucagon-like peptide 1 (GLP-1): a potent gut hormone with a possible therapeutic perspective. Acta Diabetol 35:117–129
Bharatham K, Bharatham N, Park KH, Lee KW (2008) Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors. J Mol Graph Model 26:1202–1212
Matsui T, Oki T, Osajima Y (1999) Isolation and identification of peptidic α-glucosidase inhibitors derived from sardine muscle hydrolyzate. Z Naturforsch C 54:259–263
Lavigne C, Marette A, Jacques H (2000) Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol 278:E491–E500
Lavigne C, Tremblay F, Asselin G, Jaques H, Marette A (2001) Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am J Physiol Endocrinol Metab 281:E62–E71
Huang FJ, Wu WT (2010) Purification and characterization of a new peptide (s-8300) from shark liver. J Food Biochem 34:962–970
Yu Z, Yin Y, Zhao W, Liu J, Chen F (2012) Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem 135:2078–2085
Ramchandran L, Shah NP (2009) Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme- and α-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci Technol 89:583–600
Fan H, Yan S, Stehling S, Marguet D, Schuppan D, Reutter W (2003) Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. Adv Exe Med Biol 524:165–174
Shubrook J, Colucci R, Guo A, Schwartz F (2011) Saxagliptin: a selective DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 4:1–12
Kushner P, Gorrell M (2010) DPP-4 inhibitors in type 2 diabetes: importance of selective enzyme inhibition and implications for clinical use. J Fam Pract 59(2):1
Wang XM, Yu DM, McCaughan GW (2005) Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cellline. Hepatology 42:935–945
Amerongen AV, Beelen MJC, Wolbers LAM, Gilst WH, Buikema JH, Nelissen JWPM (2009) Egg protein hydrolysates. WO 2009/128713 A1 (Patent)
Li-Chan ECY, Huang SL, Jao CL, Ho KP, Hsu KC (2012) Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60:973–978
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipeptidyl peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Velarde-Salcedo AJ, Barrera-Pacheco A, Lara-González S, Montero-Morán GM, Díaz-Gois A, Gonzá lez de Mejia E, Barba de la Rosa A (2013) In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem 136:758–764
Hatanaka T, Inoue Y, Arima J, Kumagai Y, Usuki H, Kawakami K, Kimura M, Mukaihara T (2012) Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem 134:797–802
Mochida T, Hira T, Hara H (2010) The corn protein, zein hydrolysate, administered into the ileum attenuates hyperglycemia via its dual action on glucagon-like peptide-1 secretion and dipeptidyl peptidase-IV activity in rats. Endocrinology 151:3095–3104
Tulipano G, Cocchi D, Caroli AM (2012) Comparison of goat and sheep β-lactoglobulin to bovine β-lactoglobulin as potential source of dipeptidyl peptidase IV (DPP-4) inhibitors. Int Dairy J 24:97–101
Uchida M, Ohshiba Y, Mogami O (2011) Novel dipeptidyl peptidase-4-inhibiting peptide derived from β-lactoglobulin. J Pharmacol Sci 117:63–66
Lacroix IME, Li Chan ECY (2012) Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J Funct Food 4:403–422
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: influence of fractionation, stability to simulated gastrointestinal digestion and food-drug interaction. Int Dairy J 32:33–39
Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahrèn B (2006) Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147:3173–3180
Demuth HU, McIntosh CH, Pederson RA (2005) Type 2diabetes—therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta 1751:33–44
Deacon CF (2005) What do we know about the secretion and degradation of incretin hormones? Regul Pept 128:117–124
Kim W, Egan JM (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60:470–512
Kalra S (2011) Emerging role of dipeptidyl peptidase-IV (DPP-4) inhibitor vildagliptin in the management of type 2 diabetes. J Assoc Physicians India 59:237–245
Addison D, Aguilar D (2011) Diabetes and cardiovascular disease: the potential benefit of incretin-based therapies. Curr Atheroscler Rep 13:115–122
Holst JJ, Deacon CF (2004) Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol 4:589–596
Thornberry NA, Gallwitz B (2009) Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab 23:479–486
Thoma R, Löffler B, Stihle M, Huber W, Ruf A, Hennig M (2003) Structure basis of proline specific exopeptidase activity as observed in human dipeptidyl peptidase-IV. Structure 11:947–959
Rajput R (2009) Dipeptidyl Peptidase-IV Inhibitors: a new drug in the Therapeutic Armamentarium for treatment of Type 2 Diabetes Mellitus. J Indian Acad Clin Med 10:128–133
Deacon CF (2007) Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 9:23–31
Weber AE (2004) Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem 47:4135–4141
Deacon CF, Ahren B, Holst JJ (2004) Inhibitors of dipeptidyl peptidase-IV: a novel approach for the prevention and treatment of Type 2 diabetes? Expert Opin Investig Drugs 13:1091–1102
Herman GA, Bergman A, Stevens C (2006) Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 91:4612–4619
Estrada-Salas PA, Montero-Moran GM, Martinez-Cuevas PP, Gonzalez C, Barba de la Rosa AP (2014) Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J Agric Food Chem 62(2):427–433
Pieter BJW (2006) Protein hydrolysate enriched in peptides inhibiting DPP-IV and their use. WO 2006/068480 200 (Patent)
Green BD, Flatt PR, Bailey CJ (2006) Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res 3:159–165
Zhu CF, Li GZ, Peng HB, Zhang F, Chen Y, Li Y (2010) Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl Physiol Nutr Metab 35:797–804
Guerard F, Decourcelle N, Sabourin C, Floch-Laizet C, Le Grel L, Le Floch P, Gourlay F, Le Delezir R, Jaouen P, Bourseau P (2010) Recent developments of marine ingredients for food and nutraceutical applications: a review. Journal des Sciences Halieutiques et Aquatiques 2:21–27
Tominaga Y, Yokota S, Tanaka H et al (2012) Inventors Kaneka Corporation, assignee. Dipeptidyl peptidase-4 inhibitor. United States US 2012 0189611 (Patent)
Silva-Sánchez C, de la Rosa APB, León-Galván MF (2008) Bioactive peptides in amaranth (Amaranthus hypo-chondriacus) seed. J Agric Food Chem 56:1233–1240
Hsu KC, Tung YS, Huang SL Jao CL (2013) Dipeptidyl peptidase-IV inhibitory activity of peptides in porcine skin gelatin hydrolysates. In Hernández-Ledesma B (ed) Bioactive food peptides in health and disease, pp 205–218. doi:10.5772/51264
Dziuba M, Dziuba B, Iwaniak A (2009) Milk proteins as precursors of bioactive peptides. Acta Sci Pol Technol Aliment 8:71–90
Boots J (2006) Inventor Campina Nederland Holding BV assignee. Protein hydrolysates enriched in peptides inhibiting DPP IV and thier use. WO 2006/068480 200 (Patent)
Nongonierma AB, FitzGerald RJ (2014) Susceptibility of milk protein derived peptides to dipeptidyl peptides IV (DPP-IV) hydrolysis. Food Chem 145:845–852
Silveira ST, Martínez-Maqueda D, Recio I, Hernández-Ledesma B (2013) Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem 141:1072–1077
Tremblay A, Gilbert JA (2009) Milk products, insulin resistance syndrome and type 2 diabetes. J Am Coll Nutr 28(Suppl 1):91S–102S
Phelan M, Aisling A, FitzGerald RJ, O’Brien NM (2009) Casein-derived bioactive peptides: biological effects, industrial uses, safety aspects and regulatory status. Int Dairy J 19:643–654
Gill I, López-Fandiño R, Jorba X, Vulfson EN (1996) Biologically active peptides and enzymatic approaches to their production. Enzyme Microb Technol 18:163–183
Madureira AR, Tavares T, Gomes MP, Malcata FX (2010) Physiological properties of bioactive peptides obtain from whey proteins. J Dairy Sci 93:437–455
Kilara A, Panyam D (2003) Peptides from milk proteins and their properties. Crit Rev Food Sci Nut 43:607–633
Korhonen H, Pihlanto A (2003) Food-derived bioactive peptides-opportunities for designing future foods. Curr Pharm Des 9:1297–1308
Haque E, Chand R, Kapila S (2008) Biofunctional properties of bioactive peptides of milk origin. Food Rev Int 25:28–43
Urista CM, Fernández RA, Rodríguez FR, Cuenca AA, Jurado AT (2011) Review: production and functionality of active peptides from milk. Food Sci Technol Int 17:293–317
Juillard V, Le Bars D, Kunji ER, Konings WN, Gripon JC, Richard J (1995) Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol 61:3024–3030
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76:217–246
Alsayadi M, Jawfi AL et al (2014) Evaluation of anti-hyperglycemic and anti-hyperlipidemic activities of water kefir as probiotic on streptozotocin-induced diabetic wistar rats. J Diabetes Mellit 4:85–95
Teruya K, Yamashita M et al (2002) Fermented milk, Kefram-Kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology 40:107–116
Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Gheshlaghi ZB, Vahedjabbari M (2015) Effect of probiotic fermented milk (Kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 44:228–237
Maeda H, Zhu X, Mitsuoka T (2004) Effects of an exopolysaccharide (kefiran) from Lactobacillus kefiranofaciens on blood glucose in KKAy mice and constipation in SD rats induced by a low-fiber diet. Biosci Microflora 23:149–153
Yi N, Hwang JY, Han JS (2009) Hypoglycemic effect of fermented soymilk extract in STZ-induced diabetic mice. J Food Sci Nutr 14:8–13
Yadav H, Jain S, Sinha PR (2007) Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23:62–68
Kim SK, Wijesekara I (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2:1–9
Pedroche J, Yust MM, Lqari H, Megias C, Girón-Calle J, Alaiz M (2007) Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases. Food Res Int 40:931–938
Manders RJF, Hansen D, Zorenc AHG, Dendale P, Kloek J, Saris WHM, van Loon LJC (2014) Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. J Med Food 17:758–763
Méric E, Lemieux S, Turgeon SL, Bazinet L (2014) Insulin and glucose responses after ingestion of different loads and forms of vegetable or animal proteins in protein enriched fruit beverages. J Funct Foods 10:95–103
Goudarzi M, Madadlou A (2013) Influence of whey protein and its hydrolysate on prehypertension and postprandial hyperglycaemia in adult men. Int Dairy J 33:62–66
Jonker JT, Wijngaarden MA et al (2011) Effect of low doses of casein hydrolysate on post-challenge glucose and insulin levels. Eur J Intern Med 22:245–248
Acknowledgments
The authors thank the Director of NDRI for supporting the work. There are no conflicts of interest whatsoever among the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, P., Mandal, S., Tomar, S.K. et al. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur J Nutr 54, 863–880 (2015). https://doi.org/10.1007/s00394-015-0974-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0974-2