Abstract
Purpose
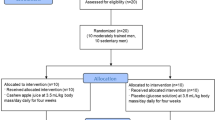
The effect of endogenous antioxidants can be either an immediate response (relying on enzymatic activities) or a long-term adaptation (relying on gene modulation events), both susceptible to be modified by antioxidants from diet and supplementation. The aim of this work was to delve in these aspects in circulating white blood cells in a group of volunteers (n = 33, 20–22 years) performing eccentric exercises and consuming or not (n = 8) different polyphenolic antioxidants (Lippia citriodora extract-PLX® n = 8, almond beverage n = 9 or a mixture of both n = 8) during 21 days.
Methods
We have designed a single-blind, parallel-group, randomized controlled trial. Antioxidant enzyme activities, oxidative stress markers, and antioxidant gene expression were determined.
Results
Neutrophils and lymphocytes expressed high amounts of oxidative markers compared to plasma. Concerning enzymatic activities, increased superoxide dismutase levels were detected when certain supplements were consumed. However, catalase levels did not change. As for glutathione peroxidase levels, no differences were detected in lymphocytes, while neutrophils expressed increased levels in both placebo and PLX® groups. Glutathione reductase activity was decreased in all groups, except in neutrophils of PLX® group. At the level of gene expression, neither PLX® nor the almond beverage interfered with the expression of genes coding for the corresponding enzymes. However, the combined intake of both supplements affected the expression of glutathione reductase and Cu–Zn and Mn-superoxide dismutases in neutrophils.
Conclusions
Altogether, these results suggest that blood cell types respond and adapt differently to exercise-induced oxidative damage.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
McGinley C, Shafat A, Donnelly AE (2009) Does antioxidant vitamin supplementation protect against muscle damage? Sports Med 39:1011–1032
Morton JP, Kayani AC, McArdle A, Drust B (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39:643–662
Smith C, Kruger MJ, Smith RM, Myburgh KH (2008) The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med 38:947–969
Toumi H, F’guyer S, Best TM (2006) The role of neutrophils in injury and repair following muscle stretch. J Anat 208:459–470
Geering B, Simon HU (2011) Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ 18:1457–1469
Tauler P, Aguilo A, Gimeno I, Noguera A, Agusti A, Tur JA, Pons A (2003) Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic Res 37:931–938
Cases N, Aguilo A, Tauler P, Sureda A, Llompart I, Pons A, Tur JA (2005) Differential response of plasma and immune cell’s vitamin E levels to physical activity and antioxidant vitamin supplementation. Eur J Clin Nutr 59:781–788
Moreira A, Delgado L, Moreira P, Haahtela T (2009) Does exercise increase the risk of upper respiratory tract infections? Br Med Bull 90:111–131
Gleeson M, Bishop NC (2000) Elite athlete immunology: importance of nutrition. Int J Sports Med 21(Suppl 1):S44–S50
Sen CK (2001) Antioxidants in exercise nutrition. Sports Med 31:891–908
Nikolaidis MG, Kerksick CM, Lamprecht M, McAnulty SR (2012) Does vitamin C and E supplementation impair the favourable adaptations of regular exercise? Oxid Med Cell Longev. doi:10.1155/2012/707941
Jakeman P, Maxwell S (1993) Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur J Appl Phys Occup Physiol 67:426–430
Shafat A, Butler P, Jensen RI, Donnelly AE (2004) Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Eur J Appl Physiol 93:196–202
Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J (2008) Oral administration of vitamin C decreases muscle mitochondria biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87:142–149
Marshall RJ, Scott KC, Hill RC, Lewis DD, Sundstrom D, Jones GL, Harper J (2002) Supplemental vitamin C appears to slow racing greyhounds. J Nutr 132(Suppl 2):1616S–1621S
Bailey DM, Williams C, Betts JA, Thompson D, Hurst TL (2011) Oxidative stress, inflammation and recovery of muscle function after damaging exercise: effect of 6-week mixed antioxidant supplementation. Eur J Appl Physiol 111:925–936
Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK (2010) Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 42:1388–1395
Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH (2011) Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 301:E779–E784
Elhaimeur F, Courderot-Masuyer C, Nicod L, Guyon C, Richert I, Berthelot A (2002) Dietary vitamin C supplementation decreases blood pressure in DOCA-salt hypertensive male Sprague-Dawley rats and this is associated with increased liver oxidative stress. Mol Cell Biochem 237:77–83
Nieman DC, Henson DA, McAnulty SR, McAnulty LS, Morrow JD, Ahmed A, Heward CB (2004) Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 36:1328–1335
McAnulty SR, McAnulty LS, Nieman DC, Morrow JD, Shooter LA, Holmes S, Heward C, Henson DA (2005) Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J Nutr Biochem 16:530–537
Close GL, Ashton T, Cable T, Doran D, Holloway C, McArdle F, McLaren DP (2006) Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr 95:976–981
Rytter E, Vessby B, Asgard R, Ersson C, Moussavian S, Sjödin A, Abramsson-Zetterberg L, Möller L, Basu S (2010) Supplementation with a combination of antioxidants does not affect glycaemic control, oxidative stress or inflammation in type 2 diabetes subjects. Free Radic Res 44:1445–1453
Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y, Jamurtas AZ (2011) No effect of antioxidant supplementation on muscle performance and blood redox adaptations to eccentric training. Am J Clin Nutr 93:1373–1383
Niess AM, Simon P (2007) Response and adaptation of skeletal muscle to exercise: the role of reactive oxygen species. Front Biosci 12:4826–4838
Bucci LR (2000) Selected herbals and human exercise performance. Am J Clin Nutr 72(Suppl 2):624S–636S
Funes L, Fernandez-Arroyo S, Laporta O, Pons A, Roche E, Segura-Carretero A, Fernandez-Gutierrez A, Micol V (2009) Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extracts. Food Chem 117:589–598
Carrera-Quintanar L, Funes L, Viudes E, Tur J, Micol V, Roche E, Pons A (2012) Antioxidant effect of lemon verbena extracts in lymphocytes of university students performing aerobic training program. Scand J Med Sci Sports 22:454–461
Funes L, Carrera-Quintanar L, Cerdan-Calero M, Ferrer MD, Drobnic F, Pons A, Roche E, Micol V (2011) Effect of lemon verbena supplementation on muscular damage markers, proinflammatory cytokines release and neutrophils’oxidative stress in chronic exercise. Eur J Appl Physiol 111:695–705
Sureda A, Tauler P, Aguilo A, Cases N, Llompart I, Tur JA, Pons A (2007) Antioxidant supplementation influences the neutrophil tocopherol associated protein expression, but not the inflammatory response to exercise. Cent Eur J Biol 2:56–70
Sureda A, Tauler P, Aguilo A, Cases N, Llompart I, Tur JA, Pons A (2008) Influence of an antioxidant vitamin-enriched drink on pre- and post-exercise lymphocyte antioxidant system. Ann Nutr Metab 52:233–240
Venkatachalam M, Sathe SK (2006) Chemical composition of selected edible nut seeds. J Agric Food Chem 54:4705–4714
Mestre-Alfaro A, Ferrer MD, Sureda A, Tauler P, Martinez E, Bibiloni MM, Micol V, Tur JA, Pons A (2011) Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur J Appl Physiol 111:2281–2294
Quirantes-Piné R, Herranz-López M, Funes L, Borrás-Linares I, Micol V, Segura-Carretero A, Fernández-Gutiérrez A (2013) Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 20:1112–1118
Marfell-Jones M, Olds T, Steward A, Carter L (2006) International standards for anthropometric assessment. ISAK, Potchefstroom
Boyum A (1964) Separation of white blood cells. Nature 204:793–794
Trinder P (1969) Determination of blood glucose using an oxidase–peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19:476–482
Fossati P, Prencipe L, Berti G (1980) Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Bowers LD, Wong ET (1980) Kinetic serum creatineine assays. II. A critical evaluation and review. Clin Chem 26:555–561
Weisshaar D, Gossrau E, Faderl B (1975) Normal ranges of alpha-HBDH, LDH, AP, and LAP as measured with substrate-optimated test charges. Med Welt 26:387–392
Young DS, Friedman RB (2001) Effects of disease on clinical laboratory tests. AACC Press, Washington DC
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Flohé L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Goldberg DM, Spooner RJ (1985) Glutathione reductase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Basel, pp 258–265
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Laporta O, Funes L, Garzon MT, Villalain J, Micol V (2007) Role of membranes on the antibacterial and anti-inflammatory activities of the bioactive compounds from hypoxis rooperi corm extract. Arch Biochem Biophys 467:119–131
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods 25:402–408
Bloomer RJ (2008) Effect of exercise on oxidative stress biomarkers. Adv Clin Chem 46:1–50
Vander A, Sherman J, Luciano D (2001) Human physiology. McGraw Hill, Boston
Hausser J, Syed AP, Selevsek N, van Nimwegen E, Jaskiewick L, Aebersold R, Zavolan M (2013) Timescales and bottlenecks in miRNA-dependent gene regulation. Mol Syst Biol 9:711
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232
Serafini M, Miglio C, Peluso I, Petrosino T (2011) Modulation of plasma non enzimatic antioxidant capacity (NEAC) by plant foods: the role of polyphenols. Curr Top Med Chem 11:1821–1846
Yamada K, Tachibana H (2000) Recent topics in anti-oxidant factors. BioFactors 13:167–172
Osawa T (1999) Protective role of dietary polyphenols in oxidative stress. Mech Ageing Dev 111:133–139
Rice-Evans C (1995) Plant polyphenols: free radical scavengers or chain-breaking antioxidants? Biochem Soc Symp 61:103–116
Joven J, Rull A, Rodríguez-Gallego E, Camps J, Riera-Borrull M, Hernández-Aguilera A, Martín-Paredero V, Segura-Carretero A, Micol V, Alonso-Villaverde C, Menéndez JA (2013) Multifunctional targets of dietary polyphenols in disease: a case for chemokine network and energy metabolism. Food Chem Toxicol 51:267–279
Bouayed J, Bohn T (2010) Exogenous antioxidants, double-edge swords in cellular redox state. Oxid Med Cell Longev 3:228–237
Sureda A, Batle JM, Tauler P, Aguiló A, Cases N, Tur JA, Pons A (2004) Hypoxia/reoxygenation and vitamin C intake influence NO synthesis and antioxidant defenses of neutrophils. Free Radic Biol Med 37:1744–1755
Acknowledgments
We thank Jose Maria Adsuar for technical assistance in blood analysis. This work was supported by grants from Spanish Science Ministry AGL2007-62806/ALI to AP, AGL2007-60778 and AGL 2011-29857-C03-03 to VM and PROMETEO/2012/007 from Generalitat Valenciana to VM and ER. ER is recipient of Instituto de Salud Carlos III-FEDER (PS09/01093) and Fundacion Salud 2000-Merck Serono grants. AP, VM and ER are members of the “Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición” CIBERobn (CB12/03/30038). LC-Q and LF were recipients of CONACYT-Mexico (ref 197139) and FPI (Spanish Science Ministry) fellowships, respectively.
Conflict of interest
Authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carrera-Quintanar, L., Funes, L., Vicente-Salar, N. et al. Effect of polyphenol supplements on redox status of blood cells: a randomized controlled exercise training trial. Eur J Nutr 54, 1081–1093 (2015). https://doi.org/10.1007/s00394-014-0785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0785-x
Keywords
Profiles
- Cristina Blasco-Lafarga View author profile