Abstract
Purpose
Tomato products are a dietary source of natural antioxidants, especially lycopene, which accumulates in the liver, where it exerts biological effects. Taking into consideration this fact, the aim of the present study was to ascertain the effect of tomato consumption on biomarkers and gene expression related to lipid metabolism in rats with induced steatosis.
Methods
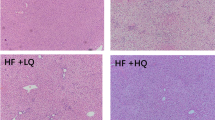
Adult male Sprague–Dawley rats (8 weeks old) were randomly grouped (n = 6 rats/group) in four experimental groups: NA (normal diet and water), NL (normal diet and tomato juice), HA (high fat diet and water) and HL (high fat diet and tomato juice). After 7 weeks, rats were euthanized, and plasma, urine, feces and liver were sampled to analyze the biomarkers related to lipid metabolism, inflammation and oxidative stress.
Results
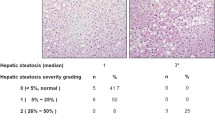
The H diet induced steatosis (grade II) in the HA and HL groups, which was confirmed by the levels of alanine aminotransferase and aspartate aminotransferase, histological examination and the presence of dyslipidemia. The intake of tomato juice led to an accumulation of all-E and Z-lycopene and its metabolites in the livers of these animals; levels were higher in HL than in NL, apparently due to higher absorption (63.07 vs. 44.45 %). A significant improvement in the plasma level of high-density lipoprotein was observed in the HL group compared with HA animals, as was an alleviation of oxidative stress through reduction of isoprostanes in the urine. In relation to fatty acid gene expression, an overexpression of several genes related to fatty acid transport, lipid hydrolysis and mitochondrial and peroxisomal β-fatty acid oxidation was observed in the HL group.
Conclusions
The consumption of tomato juice and tomato products reduced hallmarks of steatosis, plasmatic triglycerides and very low-density lipoproteins, and increased lipid metabolism by inducing an overexpression of genes involved in more efficient fatty acid oxidation.

Similar content being viewed by others
References
Charatcharoenwitthya P, Lindor KD (2010) Lipid metabolism and control in nonalcoholic fatty liver disease. In: Preedy VR, Lakshman R, Srirajaskanthan R, Watson RR (eds) Nutrition, diet therapy, and the liver. CRC Press, FL 33487-2742, USA, pp 67–80
Adiels M, Westerbacka J, Soro Paavonen A, Hakkinen AM, Vehkavaara S, Caslake MJ et al (2007) Acute suppression of VLDL (1) secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia 50:2356–2365
Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Jarvinen H, Svegliati-Baroni G (2010) From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 42:320–330
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S et al (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 59:138–143
Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S (2003) Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 98:2485–2490
Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ (2003) Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 38:413–419
Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N et al (2008) Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol 8:40
Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang X-D (2010) Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer 26:1788–1796
Ahn J, Lee H, Jung CH, Ha T (2012) Licopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol Nutr Food Res 56:1665–1674
Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N (2007) N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol 13:5127–5132
Periago MJ, García-Alonso J, Jacob K, Olivares AB, Bernal MJ, Iniesta MD et al (2009) Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int J Food Sci Nutr 60:694–708
García-Valverde V, Navarro-González I, García-Alonso J, Periago M (2013) Antioxidant Bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol 6:391–402
Garcia-Alonso FJ, Bravo S, Casas J, Pérez-Conesa D, Jacob K, Periago MJ (2009) Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. J Agric Food Chem 57:6815–6822
Jacob K, Periago MJ, Böhm V, Berruezo G (2008) Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Brit J Nutr 99:137–146
García-Alonso FJ, Jorge-Vidal V, Ros G, Periago MJ (2012) Effect of consumption of tomato juice enriched with & #x03C9;-3 polyunsaturated fatty acids on the lipid profile, antioxidant biomarker status, and cardiovascular disease risk in healthy women. Eur J Nutr 5:415–424
Bernal C, Martín-Pozuelo G, Lozano AB, Sevilla A, García-Alonso J, Canovas M et al (2013) Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J Nutr Biochem 24:1870–1881
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L et al (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC) of plasma and other biological and food samples. J Agric Food Chem 51:3273–3279
Helger R, Rindfrey H, Hilgenfeldt J (1974) Direct estimation of creatinine in serum and in urine without deproteinization using a modified Jaffé method. Z Klin Chem Klin Biochem 12:344–349
Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L (2005) Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chromatogr B 827:76–82
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70:241–250
Seybold C, Fröhlich K, Bitsch R, Otto K, Böhm V (2004) Changes in contents of carotenoids and vitamin E during tomato processing. J Agric Food Chem 52:7005–7010
Peso Echarri P, Frontela-Saseta C, Santaella-Pascual M, García-Alcaráz A, Abdel I, Ros-Berruezo G et al (2012) Sodium alginate as seed additive in cultured sea bream (Sparus aurata): does it modify the quality of the flesh? Food Chem 135:699–705
Hijona E, Hijona L, Larzabal M, Sarasqueta C, Aldazabal P, Arenas J, Bujanda L (2010) Biochemical determination of lipid content in hepatic steatosis by Soxtec method. World J Gastroenterol 16:1495–1499
León Goñi AC, Blanco D, Peña A, Ronda M, González BO, Arteaga ME et al (2011) Hematological and biochemical parameters in Sprague Dawley laboratory rats breed in CENPALAB, Cenp: SPRD. Rev Electrón Vet 12:1–10
Moreira EAM, Fagundes RLM, Wilhelm D, Neves D, Sell F, Bellisle F et al (2005) Effects of diet energy level and tomato powder consumption on antioxidant status in rats. Clin Nutr 24:1038–1046
Ahmed U, Redgrave TG, Oates PS (2009) Effect of dietary fat to produce non-alcoholic fatty liver in the rat. J Gastroenterol Hepatol 24:1463–1471
Maiani G, Periago Castón MJ, Catasta G, Toti E, Goni Cambrodon I, Bysted A et al (2009) Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 53:S194–S218
Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE et al (2011) Lycopene and cardiovascular diseases: an update. Curr Med Chem 18:1146–1163
Navarro-González I, Pérez-Sánchez H, Martín-Pozuelo G, García-Alonso J, Periago MJ (2014) The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: in vivo study and molecular modelling. PLoS ONE 9:e83968
Musso G, Gambino R, Cassader M (2009) Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res 48:1–26
Markovits N, Amotz AB, Levy Y (2009) The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. IMAJ 11:598–601
Sookoian S, Castaño GO, Burgueño AL, Rosselli MS, Fernández Gianotti T, Mallardi P et al (2010) Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 209:585–591
Roberts LJ, Morrow JD (2000) Measurement of F-2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28:505–513
Helledie T, Antonius M, Sorensen RV, Hertzel AV, Bernlohr DA, Kølvraa S et al (2000) Lipid-binding proteins modulate ligand-dependent trans-activation by peroxisome proliferators-activated receptors and localize to the nucleus as well as the cytoplasm. J Lipid Res 41:1740–1751
Wolfrum C, Borrmann CM, Borchers T, Spener F (2001) Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci USA 98:2323–2328
Tan NS, Shaw NS, Vinckenbosh N, Liu P, Yasmin R, Desvergne B et al (2002) Selective cooperation between fatty acid binding proteins and peroxisome proliferators-activated receptors in regulating transcription. Mol Cell Biol 22:5114–5127
Iseki S, Kondo H, Hitomi M, Ono T (1990) Localization of liver fatty acid-binding protein and its mRNA in the liver and jejunum of rats: an immunohistochemical and in situ hybridization study. Mol Cell Biochem 98:27–33
Carey JO, Neufer D, Farrar RP, Veerkamp JH, Dohm GL (1994) Transcriptional regulation of muscle fatty acid-binding protein. Biochem J 298:613–617
Auinger A, Helwih U, Rubin S, Herrmann J, Jahreis G, Pfeuffer M et al (2010) Human intestinal fatty acid binding protein 2 expression is associated with fat intake and polymorphisms. J Nutr 140:1411–1417
Doege H, Ra Baillie, Ortegon AM, Tsang B, Wu Q, Punreddy S et al (2006) Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology 130:1245–1258
Courtney M, Stahl A (2013) SLC27 fatty acid transport proteins. Mol Aspects Med 34:516–528
Fruchard JC, Duriez P (2006) Mode of action of fibrates in the regulation of triglycerides and HDL-cholesterol metabolism. Drugs Today 42:39–64
Wong H, Schotz MC (2002) The lipase gene family. J Lipid Res 43:993–999
Augustus AS, Kako Y, Yagyu H, Goldberg IJ (2003) Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Meta 284:E331–E339
Fernández C, Schumann K, Herzog R, Fielding B, Frayn K, Schevchenko A et al (2011) Altered desaturation and elongation of fatty acids in hormone-sensitive lipase null mice. PLoS ONE 6:e21603–e31615
Lee SEL, Lee EH, Lee TJ, Kim SW, Kim BH (2013) Anti-obesity effect and action mechanism of Adenophora triphylla root ethanol extract in C57BL/6 obese mice fed a high-fat diet. Biosci Biotechnol Biochem 77:544–550
Gyamfi D, Patel V (2010) Liver metabolism: biochemical and molecular regulations. In: Preedy VR, Lakshman R, Srirajaskanthan R, Watson RR. Nutrition, diet therapy, and the liver. CRC Press, FL 33487-2742, USA, pp 3–15
Ramírez-Torres A, Barceló-Batllori S, Fernández-Vizarra E, Navarro MA, Amal C, Guillén N et al (2012) Proteomics and gene expression analyses of mitochondria from squalene-treated apoE-deficient mice identify short-chain specific acyl-CoA dehydrogenase changes associated with fatty liver amelioration. J Proteomics 75:2563–2575
Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T et al (2007) Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 20:351–358
Tomonaga T, Masushite K, Yamagichi S, Oh-Ishi M, Kodera Y, Maeda T et al (2004) Identification of altered protein expression and post-translational modifications in primary colorectal cancer by using agarose two-dimensional gel electrophoresis. Clin Can Res 10:2007–2014
Bijland S, Mancini SJ, Salt LP (2013) Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci 124:491–507
Palozza P, Simone R, Catalano A, Monego G, Barini A, Mele MC et al (2011) Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. J Nutr Biochem 22:259–268
Acknowledgments
This research was supported by the projects MINECO (Spanish)/FEDER-EU BIO2012-38103 and CONSOLIDER Fun-C-Food CSD2007, as well as by the Research Regional Agency “Fundación Séneca” (Murcia, Spain) Project 12031/PI/09. We would also like to thank Zumos Hesperia Filab and Juver Alimentación (Cabezo de Torres, Murcia, Spain) for providing the tomato juice samples. R G-B thanks Spanish MICINN for the post-doctoral contract (Juan de la Cierva Program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín-Pozuelo, G., Navarro-González, I., González-Barrio, R. et al. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur J Nutr 54, 933–944 (2015). https://doi.org/10.1007/s00394-014-0770-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0770-4