Abstract
Background
In previous studies we reported an altered prostanoid (PR) release-pattern in mesenteric vessels in fructose (F)-overloaded rats, an experimental model of insulin resistance and hypertension. Dehydroepiandrosterone (DHEA) and its precursor Dehydroepiandrosterone sulfate (DHEA-S) are the most abundant circulating steroid hormones produced by the adrenal and recent studies in both cells and animals suggest that DHEA may have acute non-genomic actions that mimic both metabolic and vascular actions of insulin.
Aim of the study
This study was to analyze in F-overloaded rats, the effects of DHEA treatment on arterial blood pressure and the PR production in mesenteric vessels and aorta.
Methods
Male 6 week-old Sprague–Dawley rats were randomly divided in four groups: a control group (C), a DHEA (30 mg/kg/sc/48 h)-treated group (D), a fructose (10% w/v in drinking water)-fed group (F), and both treatments simultaneously group (FD). The systolic blood pressure (SBP) was measured by tail cuff method and glycemia and triglyderidemia were measured by enzymatic assays. The mesenteric beds of all groups were dissected, and incubated in Krebs solution. The PR released were measured by HPLC.
Results
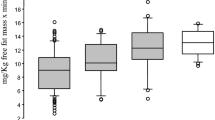
F overload increased SBP and triglyceridemia and decreased the mesenteric vasodilatory PR release. DHEA treatment prevented the increment in SBP and triglyceridemia and decreased vasoconstrictor PR in F-treated rats.
Conclusion
DHEA normalize the PGI2/TX ratio, diminished in F-overloaded rats, through the decrease in thromboxane (TX) production and this could be one of the mechanisms by which DHEA prevented the slight hypertension in F-animals.





Similar content being viewed by others
References
Akarasereenont P, Techatraisak K, Thaworn A, Chotewuttakorn S (2000) The induction of cyclooxygenase-2 by 17-beta-estradiol in endothelial cells is mediated through protein kinase C. Inflamm Res 49:460–465
Argano M, Parola S, Brignardello E, Manti R, Betteto S, Tamagno E, Danni O, Boccuzzi G (2001) Oxidative stress and eicosanoids in the kidneys of hyperglycemic rats treated with dehydroepiandrosterone. Free Radical Biol Med 31:935–942
Barret-Connor E, Kaw KT, Yen SS (1986) A prospective study of dehydroepiandrosterone sulphate, mortality, and cardiovascular disease. N Engl J Med 315:1519–1524
Baulieu EE, Corpechot C, Dray F, Emiliozzi R, Lebeau MC, Mauvais-Jarvis P, Robel P (1965) An adrenal secreted androgen: dehydroepian- drosterone sulfate. Its metabolism and a tentative generalization on the metabolism of other steroid conjugates in man. Recent Prog Horm Res 21:411–500
Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F (1994) Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab 79:1086–1090
Bonnet S, Dumas-de-la-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, et al (2003) Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. PNAS 100:9488–9493
Chistensen KL, Mulvany MJ (2001) Location of resistance arteries. J Vasc Res 38:1–12
Damiano PF, Cavallero S, Mayer M, Rosón MI, de la Riva I, Fernández B, Puyó AM (2002) Impaired response to insulin associated to protein kinase C in chronic fructose-induced hypertension. Blood Press 11:345–351
Dhatariya K, Bigelow ML, Nair KS (2005) Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes 54:765–769
Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, FritzGerald GA (2004) COX-2-derived prostacyclin confers atheroprotection on female mice. Science 306:1954–1956
Erdos B, Miller AW, Busija DW (2002) Impaired endothelium-mediated relaxation in isolated cerebral arteries from insulin-resistant rats. Am J Physiol 282:H2060–H2065
Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ (2006) DHEA mimics acute actions of insulin to simulate production of both NO and ET-1 via distinct PI 3-kinase- and MAP-kinase- dependent pathways in vascular endothelium. Mol Endocrinol 20:1153–1163
Galipeau D, Arikawa E, Sokivov J, McNeill SH (2001) Chronic thromboxane synthase inhibition prevents fructose-fed hypertension. Hypertension 38:872–876
Hansen PA, Han DH, Nolte LA, Chen M, Holloszy JO (1997) DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol 273:R1704–R1708
Harati M, Ani M (2004) Vanadyl sulfate ameliorates insulin resistance and restores plasma dehydroepiandrosterone-sulfate levels in fructose-fed, insulin-resistant rats. Clin Biochem 37:694–697
Holman RT, Johnson SB, Gerrard JM, Mauer SM, Kupcho-Sandberg S, Brown DM (1983) Arachidonic acid deficiency in streptozotocin-induced diabetes. Proc Natl Acad Sci USA 80:2375–2379
Hwang JS, Ho H, Hoffman BB, Reaven GM (1987) Fructose induced insulin resistance and hypetension in rats. Hypertension 10:512–516
Iamberts SW, van den Beld AW, van der Lely AJ (1997) The endocrinology of aging. Science 278:419–424
Jesse RL, Loesser K, Eich DM, Qian YZ, Nestler JE (1995) Dehydroepiandrosterone inhibits human platelet aggregation in vitro and in vivo. Ann NY Acad Sci 774:281–290
Johannes CB, Stellato RK, Feldman HA, Longcope C, McKinlay JB (1999) Relation of dehydroepiandrosterone and dehydroepiandrosterone sulfate with cardiovascular disease risk factors in women: longitudinal results from the Massachusetts women’s health study. J Clin Epidemiol 52:95–103
Karbowska J, Kochan Z (2005) Effect of DHEA on endocrine functions of adipose tissue, the involvement of PPARγ. Biochem Pharm 70:249–257
Liu D, Dillon JS (2002) Dehydroepiandrosterone activates endothelial cell nitric oxide synthase by a specific plasma membrane receptor coupled to Gαi2,3. J Biol Chem 277:21379–21388
Mastrocola R, Argano M, Betteto S, Brignardello E, Catalano MG, Danni O, Boccuzzi G (2003) Pro-oxidant effect of dehydroepiadrosterone in rats is mediated by PPAR activation. Life Sci 73:289–299
Matsuda M, DeFronzo R (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing. Diab Care 22:1462–1470
Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K (2007) Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLEFT rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 293:41480–41490
Mayer MA, Höcht C, Opezzo JA, Taira CA, Fernández BE, Puyó AM (2007) High fructose diet increases anterior hypothalamic alpha 2-adrenoceptors responsiveness. Neurosci Lett 423:128–132
Miatello R, Risler N, Gonzalez S, Castro C, Ruttler M, Cruzado M (2002) Effects of enalapril on the vascular wall in an experimental model of syndrome X. Am J Hypertens 15:872–878
Mohan PF, Ihnen JS, Levin BE, Cleary MP (1990) Effects of dehydroepiandrosterone treatment in rats with diet-induced obesity. J Nutr 120:1103–1114
Montagnani M, Chen H, Barr VA, Quon MJ (2001) Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276:30392–303928
Muniyappa R, Montagnani M, Koh KK, Quon MJ (2007) Cardiovascular actions of insulin. Endocr Rev 28:463–491
Nagal Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kasiwagi A (2002) Amelioration of high fructose-induced metabolic derangements by activation of PPARα. Am J Physiol 282:E1180–E1190
Nestler JE, Clore JN, Blackard WG (1991) Metabolism and actions of dehydroepiandrosterone in humans. J Steroid Biochem Mol Biol 40:599–605
Nestler JE, Usiskin KS, Barlascini CO, Welty DF, Clore JN, Blackard WG (1989) Suppression of serum dehydroepiandrosterone sulfate levels by insulin: an evaluation of possible mechanisms. J Clin Endocrinol Metab 69:1040–1046
Peredo HA (2001) Prostanoid release, constrictor responses to noradrenaline in the mesenteric vascular bed in non-insulin-dependent diabetes mellitus. J Auton Pharmacol 21:131–137
Peredo HA, Mayer MA, Carranza A, Puyó AM (2008) Pioglitazone and losartan prevent hypertension and hypertrigliceridemia and modify vascular prostanoids in fructosa-overloaded rats. Clin Exp Hypertens (in press)
Peredo HA, Mayer MA, Rodríguez-Fermepín M, Grinspon D, Puyó AM (2006) Oral treatment and in vitro incubation with fructose modify vascular prostanoid production in the rat. Auton Autacoid Pharmacol 26:15–20
Puyo AM, Mayer MA, Cavallero S, Donoso AS, Peredo HA (2008) Fructose overload modifies vascular morphology and prostaglandin production in rats. Clin Exp Hypertens 30:159–169
Pérez de Heredia F, Cerezo D, Zamora S, Garaulet M (2007) Effect of dehydroepiandrosterone on protein and fat digestibility, body protein and muscular composition in high-fat-diet-fed old rats. Br J Nutr 97:464–470
Reaven GM (1988) Banting lecture 1988: role of insulin resistance in human disease. Diabetes 37:1507–1597
Reaven GM, Ho H (1991) Sugar-induced hypertension in Sprague–Dawley rats. Am J Hypertens 4:610–614
Rettori V, Gimeno M, Lyson K, McCaan S (1992) Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci USA 89:11543–11546
Segal MS, Gollub E, Johnson RJ (2007) Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur J Nutr 46(7):406–417
Setty B, Stuart M (1986) 15-Hydrixy-5,8,11,13-eicosatetraenoic acids inhibits human vascular cyclooxygenase, potential role in diabetic vascular disease. J Clin Invest 77:202–211
Shafagoj Y, Opoku J, Qureshi D, Regelson W, Kalimi M (1992) Dehydroepiandrosterone prevents dexamethasone-induced hypertension in rats. Am J Physiol 263:E210–E213
Simoncini T, Hafezi-Mogham A, Brazil DP, Ley K, Chin WW, Liao JK (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-kinase . Nature 407:538–541
Thornburn W, Storlien LH, Jendius AB, Khouri S (1989) Fructose-induced insulin resistance and elevated plasma triglycerides in rats. Am J Clin Nutr 49:1155–1163
Verma S, Bhanot S, Yao J, McNeill JH (1996) Defective endothelium-dependent relaxation in fructose-hypertensive rats. Am J Hypertens 9:370–376
Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, Sheng L, Shi Y, Zhang Y (2007) Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem 18:64–72
Yamaguchi Y, Tanaka S, Yamakawa T, Yamakawa T, Kimura M, Ukawa K et al (1988) Reduced serum dehydroepiandrosterone levels in diabetic patients with hyperinsulinaemia. Clin Endocrinol (Oxf) 49:377–383
Yorek JA, Coppey LJ, Gellett JS, Davidson EP, King X, Lund DD, Dillon JS (2002): Effect of treatment of diabetic rats with dehydroepiandrosterone on vascular and neural function. Am J Physiol Endocrinol Metab 283:E1067–E1075
Yoshimata T, Yoneyama A, Jin-no Y, Tamai N, Kamiya Y (1999) Effects of dehydroepiandroseterone on mitogen-activated protein kinase in human aortic smooth muscle cells. Life Sci 65:431–440
Acknowledgments
This study was suppored by grants from the Secretaría de Ciencia y Técnica of the Universidad de Buenos Aires (Code B 109) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), PIP 2109/00 and PIP 5497. We gratefully acknowledge the valuable assistance of Dr. Belisario Fernandez in discussing and revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peredo, H.A., Mayer, M., Faya, I.R. et al. Dehydroepiandrosterone (DHEA) prevents the prostanoid imbalance in mesenteric bed of fructose-induced hypertensive rats. Eur J Nutr 47, 349–356 (2008). https://doi.org/10.1007/s00394-008-0734-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-008-0734-7