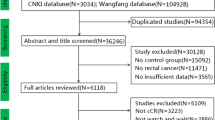
Abstract
Background
A watch-and-wait (WW) strategy or surgery for low to intermediate rectal cancer that has reached clinical complete remission (cCR) after neoadjuvant chemotherapy (nCRT) or total neoadjuvant therapy (TNT) has been widely used in the clinic, but both treatment strategies are controversial.
Objective
The aim of this study was to compare the oncologic outcomes of a watch-and-wait strategy or a surgical approach to treat rectal cancer in complete remission and to report the evidence-based clinical advantages of the two treatment strategies.
Methods
Seven national and international databases were searched for clinical trials comparing the watch-and-wait strategy with surgical treatment for oncological outcomes in patients with rectal cancer in clinical complete remission.
Results
In terms of oncological outcomes, there was no significant difference between the watch-and-wait strategy and surgical treatment in terms of overall survival (OS) (HR = 0.92, 95% CI (0.52, 1.64), P = 0.777), and subgroup analysis showed no significant difference in 5-year disease-free survival (5-year DFS) between WW and both local excision (LE) and radical surgery (RS) (HR = 1.76, 95% CI (0.97, 3.19), P = 0.279; HR = 1.98, 95% CI (0.95, 4.13), P = 0.164), in distant metastasis rate (RR = 1.12, 95% CI (0.73, 1.72), P = 0.593), mortality rate (RR = 1.62, 95% CI (0.93, 2.84), P = 0.09), and organ preservation rate (RR = 1.05, 95% CI (0.94, 1.17), P = 0.394) which were not statistically significant and on the outcome indicators of local recurrence rate (RR = 2.09, 95% CI (1.44, 3.03), P < 0.001) and stoma rate (RR = 0.35, 95% CI (0.20, 0.61), P < 0.001). There were significant differences between the WW group and the surgical treatment group.
Conclusion
There were no differences in OS, 5-year DFS, distant metastasis, and mortality between the WW strategy group and the surgical treatment group. The WW strategy did not increase the risk of local recurrence compared with local resection but may be at greater risk of local recurrence compared with radical surgery, and the WW group was significantly better than the surgical group in terms of stoma rate; the WW strategy was evidently superior in preserving organ integrity compared to radical excision. Consequently, for patients who exhibit a profound inclination towards organ preservation and the evasion of stoma formation in the scenario of clinically complete remission of rectal cancer, the WW strategy can be contemplated as a pragmatic alternative to surgical interventions. It is, however, paramount to emphasize that the deployment of such a strategy should be meticulously undertaken within the ambit of a multidisciplinary team’s management and within specialized centers dedicated to rectal cancer management.












Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
All data included in this study are available upon request by contacting the corresponding author.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Shin F, Takayuki A, Junki M et al (2012) Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 13(6)
Dossa F, Acuna SA, Rickles AS et al (2018) Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol 4:930–937
Benson AB, Venook AP, Al-Hawary MM et al (2018) Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16(7):874–901
Glynne-Jones R, Wyrwicz L, Tiret E et al (2018) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Suppl 4):iv263
Cerdán-Santacruz C, Vailati BB, São JGP et al (2022) Watch and wait: why, to whom and how. Surg Oncol 43:101774
McDermott FD, Heeney A, Kelly ME et al (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102:462–479
Blumetti J, Chaudhry V, Cintron JR et al (2014) Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg 38:985–991
Chiarello MM, Fransvea P, Cariati M et al (2022) Anastomotic leakage in colorectal cancer surgery. Surg Oncol 40:101708
Emmertsen KJ, Chen TYT, Laurberg S (2014) Functional results after treatment for rectal cancer. J Coloproctology 34:55–61
Paun BC, Cassie S, Maclean AR (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251(5):807–818
Fang Q, Li Z, Xinmei Y et al (2018) Analysis of sexual life status and influencing factors of young and middle-aged people with enterostomy. J Guangdong Med 39(39):2951–2955
Tan S, Ou Y, Huang S et al (2023) Surgical, oncological, and functional outcomes of local and radical resection after neoadjuvant chemotherapy or chemoradiotherapy for early- and mid-stage rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 38:132
Habr-Gama A, Perez RO, Nadalin W et al (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240(4)
Bao QR, Ferrari S, Capelli G et al (2023) Rectal sparing approaches after neoadjuvant treatment for rectal cancer: a systematic review and meta-analysis comparing local excision and watch and wait. Cancers (Basel) 15(2)
Li J, Ma Y, Wen L et al (2023) Outcomes after the watch-and-wait strategy and local excision treatment for rectal cancer: a meta-analysis. Expert Rev Anticancer Ther 23:555–564
Page MJ, Moher D, Bossuyt PM et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160
Huang CM, Huang CW, Ma CJ et al (2020) Predictive value of FOLFOX-based regimen, long interval, hemoglobin levels and clinical negative nodal status, and postchemoradiotherapy CEA levels for pathological complete response in patients with locally advanced rectal cancer after neoadjuvant chemoradiotherapy. J Oncol 2020:9437684
Wu A, Gao C et al (2020) Expert consensus on wait-and-see strategy after neoadjuvant therapy for rectal cancer (2020 edition)[J]. Chin J Gastrointes Surg 23:1:1–9
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al (2023) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [EB/OL]
Yanjiao MA, Xiaofan C (2022) Stata software in the application of meta system analysis. J Digital Technol Appl 40(5):34–36
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10:37–48
Thilarajah S, Mentiplay BF, Bower KJ et al (2018) Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil 99:1876–1889
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Wang L, Zhang XY, Zhao YM et al (2023) Intentional watch and wait or organ preservation surgery following neoadjuvant chemoradiotherapy plus consolidation CAPEOX for MRI-defined low-risk rectal cancer: findings from a prospective phase 2 trial (PKUCH-R01 trial, NCT02860234). Ann Surg 277:647–654
Al-Najami I, Jones HJ, Dickson EA et al (2021) Rectal cancer: watch-and-wait and continuing the rectal-preserving strategy with local excision for incomplete response or limited regrowth. Surg Oncol 37:101574
Jimenez-Rodriguez RM, Quezada-Diaz F, Hameed I et al (2021) Organ preservation in patients with rectal cancer treated with total neoadjuvant therapy. Dis Colon Rectum 64:1463–1470
Wang QX, Zhang R, Xiao WW et al (2021) The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol 16:16
Lee JK, Cho JR, Song KS et al (2021) Oncologic comparison between nonradical management and total mesorectal excision in good responders after chemoradiotherapy in patients with mid-to-low rectal cancer. Ann Surg Treat Res 101:93–101
Beard BW, Rettig RL, Ryoo JJ et al (2020) Watch-and-wait compared to operation for patients with complete response to neoadjuvant therapy for rectal cancer. J Am Coll Surg 231:681–692
Yeom SS, Lee Soo Y, Kim CH et al (2019) Non-operative treatment outcome for rectal cancer patient with clinical complete response after neoadjuvant chemoradiotherapy. Asian J Surg 42:823–831
Smith JJ, Strombom P, Chow OlS et al (2019) Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol 5:e185896
Neşşar G, Demirbağ AE, Mısırlıoğlu HC et al (2019) “Watch and wait” approach in rectal cancer patients following complete clinical response to neoadjuvant chemoradiotherapy does not compromise oncologic outcomes. Turk J Gastroenterol 30:951–956
Asoglu O, Tokmak H, Bakir B et al (2020) The impact of total neo-adjuvant treatment on nonoperative management in patients with locally advanced rectal cancer: the evaluation of 66 cases. Eur J Surg Oncol 46:402–409
Creavin B, Ryan E, Martin ST et al (2017) Organ preservation with local excision or active surveillance following chemoradiotherapy for rectal cancer. Br J Cancer 116:169–174
Vaccaro CA, Yazyi FJ, Ojra QG et al (2016) Locally advanced rectal cancer: preliminary results of rectal preservation after neoadjuvant chemoradiotherapy. Cir Esp 94:274–279
Martens MH, Maas M, Heijnen LA et al (2016) Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst 108–112
Araujo ROC, Valadão M, Borges D et al (2015) Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol 41:1456–1463
Lai CL, Lai MJ, Wu CC et al (2016) Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int J Colorectal Dis 31:413–419
Smith RK, Fry RD, Mahmoud NN et al (2015) Surveillance after neoadjuvant therapy in advanced rectal cancer with complete clinical response can have comparable outcomes to total mesorectal excision. Int J Colorectal Dis 30:769–774
Lee SY, Kim CH, Kim YJ et al (2015) Oncologic outcomes according to the treatment strategy in radiologic complete responders after neoadjuvant chemoradiation for rectal cancer. Oncology 89:311–318
Smith JD, Ruby JA, Goodman KA et al (2012) Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 256:965–972
Benson AB, Venook A, Al-Hawary MM et al (2020) NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw 18:806–815
Hongyin Z, Wenlong HE (2017) Application and sensitivity prediction of preoperative chemoradiotherapy in patients with locally advanced colorectal cancer. Chin J Gen Surg 26(3):380–385
Fernandez-Martos C, Garcia-Albeniz X, Pericay C et al (2015) Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol 26(8):1722–1728
Cotte E, Passot G, Decullier E et al (2016) Pathologic response, when increased by longer interval, is a marker but not the cause of good prognosis in rectal cancer: 17-year follow-up of the Lyon R90–01 randomized trial. Int J Radiat Oncol Biol Phys 94(3):544–553
Hanly AM, Ryan EM, Rogers AC et al (2014) Multicenter evaluation of rectal cancer reimaging post neoadjuvant (MERRION) therapy. Ann Surg 259(4):723–727
Brown CL, Ternent CA, Thorson AG et al (2003) Response to preoperative chemoradiation in stage II and III rectal cancer. Dis Colon Rectum 46:1189–1193
Appelt JP et al (2015) High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 16(8):919–927
Habr-Gama A, Perez RO, Nadalin W et al (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240(4):711; discussion 717–718
Bipat S, Glas AS et al (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging-a meta-analysis. Radiology 232(3):773–783
Perez K, Safran H, Sikov W et al (2017) Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE study. Am J Clin Oncol 40:283–287
Xiao Y, Sun R, Xu L (2019) Clinical complete response after neoadjuvant therapy for rectal cancer bed evaluation. Chin J Dig Surg 18(8):726–730
Celerier B, Denost Q, Van Geluwe B, Pontallier A, Rullier E (2016) The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Color Dis 18:59–66
Back E, Haggstrom J, Holmgren K, Haapamaki M, Matthiessen P, Rutegard J, Rutegard M (2021) Permanent stoma rates after anterior resection for rectal cancer: risk prediction scoring using preoperative variables. Br J Surg 108:1388–1395
Grumann MM, Noack EM, Hoffmann IA et al (2001) Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 233:149–156
Gunjur A, Chazan G, Newnham G, McLachlan SA (2021) Pilot study of patients’ preferences for immediate resection versus a watch and wait approach after neoadjuvant chemoradiation for locally advanced rectal cancer. J Oncol Pract 17(2):e149–e157
Yu G, Lu W, Jiao Z et al (2021) A meta-analysis of the watch-and-wait strategy versus total mesorectal excision for rectal cancer exhibiting complete clinical response after neoadjuvant chemoradiotherapy. World J Surg Oncol 19:305
Renehan AG, Malcomson L, Emsley R et al (2016) Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 17(2):174–183
Attaallah W, Ertekin SC, Yegen C (2018) Prospective study of sexual dysfunction after proctectomy for rectal cancer. Asian J Surg 41:454–461
Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM et al (2020) Patient-reported bowel function in patients with rectal cancer managed by a watch-and-wait strategy after neoadjuvant therapy: a case-control study. Dis Colon Rectum 63:897–902
Didailler R, Denost Q, Loughlin P, Chabrun E, Ricard J, Picard F, Zerbib F, Rullier E (2018) Antegrade enema after total mesorectal excision for rectal cancer: the last chance to avoid definitive colostomy for refractory low anterior resection syndrome and fecal incontinence. Dis Colon Rectum 61(6):667–672
Pape E, Decoene E, Debrauwere M, Van Nieuwenhove Y, Pattyn P, Feryn T, Pattyn PRL, Verhaeghe S, Van Hecke A, Belgian LCG (2022) The trajectory of hope and loneliness in rectal cancer survivors with major low anterior resection syndrome: a qualitative study. Eur J Oncol Nurs 56:102088
Asoglu O, Tokmak H, Bakir B et al (2020) The impact of total neo-adjuvant treatment on nonoperative management in patients with locally advanced rectal cancer: the evaluation of 66 cases. Eur J Surg Oncol 46(3):402–409
Chadi SA, Malcomson L, Ensor J, Riley RD, Vaccaro CA, Rossi GL, Daniels IR, Smart NJ, Osborne ME, Beets GL et al (2018) Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol 3:825–836
Juliao GS, Karagkounis G, Fernandez L, Habr-Gama A, Vailati B, Dattani M, Kalady M, Perez RO (2020) Conditional survival in patients with rectal cancer and complete clinical response managed by watch and wait after chemoradiation: recurrence risk over time. Ann Surg 272:138–144
Heald RJ, Beets G, Carvalho C (2014) Report from a consensus meeting: response to chemoradiotherapy in rectal cancer - predictor of cure and a crucial new choice for the patient: on behalf of the Champalimaud 2014 Faculty for ‘Rectal cancer: when NOT to operate.’ Colorectal Dis 16(5):334–337
Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88(4):822–828
van der Valk MJM, Hilling DE, Bastiaannet E et al (2018) Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391(10139):2537–2545
Cerdan-Santacruz C, Vailati B, Juliao GS, Habr-Gama A, Perez R (2022) Watch and wait: why, to whom and how. Surg Oncol 43:101774
Fernandez LM, São Julião GP, Figueiredo NL et al (2021) Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol 22(1):43–50
Smith JJ, Chow OS, Gollub MJ et al (2015) Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 15:767
Cercek A, Goodman KA, Hajj C et al (2014) Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw 12:513–519
Glynne-Jones R, Wyrwicz L, Tiret E et al (2018) Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Suppl 4)
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Author information
Authors and Affiliations
Contributions
Conceptualization: Shufa Tan; Methodology: Shufa Tan, Yaping Cui, Qiangqiang Gao,Yan Ou, Shuilan Huang; Formal analysis and investigation: Shufa Tan, YaPing Cui,Yan Ou; Writing-original draft preparation: Shufa Tan,Qiangqiang Gao; Writing—review and editing: Shufa Tan; Funding acquisition: Wenzhe Feng; Resources: Shufa Tan; Supervision: Wenzhe Feng.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval was required for this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, S., Gao, Q., Cui, Y. et al. Oncologic outcomes of watch-and-wait strategy or surgery for low to intermediate rectal cancer in clinical complete remission after adjuvant chemotherapy: a systematic review and meta-analysis. Int J Colorectal Dis 38, 246 (2023). https://doi.org/10.1007/s00384-023-04534-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04534-2