Abstract
Purpose
Computer-aided diagnosis systems for polyp characterization are commercially available but cannot recognize subtypes of sessile lesions. This study aimed to develop a computer-aided diagnosis system to characterize polyps using non-magnified white-light endoscopic images.
Methods
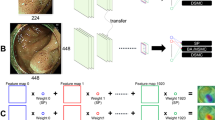
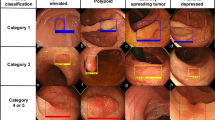
A total of 2249 non-magnified white-light images from 1030 lesions including 534 tubular adenomas, 225 sessile serrated adenoma/polyps, and 271 hyperplastic polyps in the proximal colon were consecutively extracted from an image library and divided into training and testing datasets (4:1), based on the date of colonoscopy. Using ResNet-50 networks, we developed a classifier (1) to differentiate adenomas from serrated lesions, and another classifier (2) to differentiate sessile serrated adenoma/polyps from hyperplastic polyps. Diagnostic performance was assessed using the testing dataset. The computer-aided diagnosis system generated a probability score for each image, and a probability score for each lesion was calculated as the weighted mean with a log10-transformation. Two experts (E1, E2) read the identical testing dataset with a probability score.
Results
The area under the curve of classifier (1) for adenomas was equivalent to E1 and superior to E2 (classifier 86%, E1 86%, E2 69%; classifier vs. E2, p < 0.001). In contrast, the area under the curve of classifier (2) for sessile serrated adenoma/polyps was inferior to both experts (classifier 55%, E1 68%, E2 79%; classifier vs. E2, p < 0.001).
Conclusion
The classifier (1) developed using white-light images alone compares favorably with experts in differentiating adenomas from serrated lesions. However, the classifier (2) to identify sessile serrated adenoma/polyps is inferior to experts.




Similar content being viewed by others
References
Bettington M, Walker N, Clouston A et al (2013) The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 62:367–386. https://doi.org/10.1111/his.12055
Rosty C, Hewett DG, Brown IS et al (2013) Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol 48:287–302. https://doi.org/10.1007/s00535-012-0720-y
Pai RK, Mäkinen MJ, Rosty C (2019) Colorectal serrated lesions and polyps. In: WHO Classification of Tumours Editorial Board (ed). Digestive system tumours. IARC: Lyon, France, pp 163–70
Snover DC, Ahnen DJ, Burt RW et al (2010) Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors.WHO classification of tumours of the digestive system. IARC: Lyon, France, pp 160–165
Kashida H (2019) Endoscopic diagnosis of sessile serrated polyp: A systematic review. Dig Endosc 31:16–23. https://doi.org/10.1111/den.13263
IJespeert JE, Bastiaansen BA, van Leerdam ME et al (2016) Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut 65:963–970. https://doi.org/10.1136/gutjnl-2014-308411
Kobayashi S, Yamada M, Takamaru H et al (2019) Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J 7:914–923. https://doi.org/10.1177/2050640619845987
Glover B, Teare J, Patel N (2019) A Review of new and emerging techniques for optical diagnosis of colonic polyps. J Clin Gastroenterol 53:495–506. https://doi.org/10.1097/MCG.0000000000001222
Togashi K, Nemoto D, Utano K et al (2016) Blue laser imaging endoscopy system for the early detection and characterization of colorectal lesions: a guide for the endoscopist. Therap Adv Gastroenterol 9:50–56. https://doi.org/10.1177/1756283X15603614
Rastogi A, Keighley J, Singh V et al (2009) High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol 104:2422–2430. https://doi.org/10.1038/ajg.2009.403
Ignjatovic A, East JE, Guenther T et al (2011) What is the most reliable imaging modality for small colonic polyp characterization? Study of white-light, autofluorescence, and narrow-band imaging. Endoscopy 43:94–99. https://doi.org/10.1055/s-0030-1256074
Komeda Y, Handa H, Watanabe T et al (2017) Computer-aided diagnosis based on convolutional neural network system for colorectal polyp classification: preliminary experience. Oncology 93(Suppl 1):30–34. https://doi.org/10.1159/000481227
Chen PJ, Lin MC, Lai MJ et al (2018) Accurate classification of diminutive colorectal polyps using computer-aided analysis. Gastroenterology 154:568–575. https://doi.org/10.1053/j.gastro.2017.10.010
Byrne MF, Chapados N, Soudan F et al (2019) Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 68:94–100. https://doi.org/10.1136/gutjnl-2017-314547
Kudo SE, Misawa M, Mori Y et al (2020) Artificial intelligence-assisted system improves endoscopic identification of colorectal neoplasms. Clin Gastroenterol Hepatol 18:1874-1881.e2. https://doi.org/10.1016/j.cgh.2019.09.009
Zachariah R, Samarasena J, Luba D et al (2020) Prediction of polyp pathology using convolutional neural networks achieves “resect and discard” thresholds. Am J Gastroenterol 115:138–144. https://doi.org/10.14309/ajg.0000000000000429
No author (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003(58):S3-43. https://doi.org/10.1016/s0016-5107(03)02159-x
Kaiming H, Zhang X, Ren S et al (2016) “Deep residual learning for image recognition.” In Proceedings of the IEEE conference on computer vision and pattern recognition, pp. 770–778
Deng J, Socher R, Li L et al (2009) ImageNet: A large-scale hierarchical image database. 2009 IEEE Conference on Computer Vision and Pattern Recognition
Guo Z, Zhang R, Li Q et al (2020) Reduce false-positive rate by active learning for automatic polyp detection in colonoscopy videos. Proc. IEEE International Symposium on Biomedical Imaging (ISBI'20)
Lin TY, Goyal P, Girshick R et al (2020) Focal loss for dense object detection. IEEE Trans Pattern Anal Mach Intell 42:318–327. https://doi.org/10.1109/TPAMI.2018.2858826
Zhou D, Tian F, Tian X et al (2020) Diagnostic evaluation of a deep learning model for optical diagnosis of colorectal cancer. Nat Commun 11:2961. https://doi.org/10.1038/s41467-020-16777-6
Parikh ND, Chaptini L, Njei B et al (2016) Diagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysis. Endoscopy 48:731–739. https://doi.org/10.1055/s-0042-107592
Vennelaganti S, Cuatrecasas M, Vennalaganti P et al (2021) Interobserver agreement among pathologists in the differentiation of sessile serrated from hyperplastic polyps. Gastroenterology 160:452–454e1. https://doi.org/10.1053/j.gastro.2020.09.015
Collins GS, Reitsma JB, Altman DG et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD Statement. Br J Surg 102:148–158. https://doi.org/10.1002/bjs.9736
Acknowledgements
The Statistical Consulting Service at Health Data Science Research Section, Tokyo Metropolitan Institute of Gerontology, and Clinical Research Center at Fukushima Medical University, in particular Dr. Noriko Tanaka, helped with the interpretation of the results from statistical analysis in this study. Dr. Hiroshi Hojo, former professor at Aizu Medical Center Fukushima Medical University helped with the interpretation of the histological assessment.
Author information
Authors and Affiliations
Contributions
Study conception and design: Nemoto, Guo, Hayashi, Utano, Aizawa, Zhu, Togashi. Acquisition of data: Nemoto, Aizawa, Utano, Nakajima, Hayashi, Yamashina, Togashi. Interpretation of data: Guo, Peng, Zhang, Zhu, Nemoto, Togashi. Drafting of the manuscript: Nemoto, Guo, Zhu, Togashi. Critical revision of the manuscript for important intellectual content: Nemoto, Guo, Peng, Zhang, Zhu, Aizawa, Nakajima, Utano, Hayashi, Yamashina, Lefor, Togashi. Statistical analysis: Guo, Zhu, Nemoto, Togashi. Obtained funding: Zhu, Togashi. Administrative, technical, or material support: Zhu, Togashi. Study supervision: Zhu, Togashi. Final manuscript approval: Nemoto, Guo, Peng, Zhang, Aizawa, Nakajima, Utano, Hayashi, Yamashina, Lefor, Zhu,Togashi.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nemoto, D., Guo, Z., Peng, B. et al. Computer-aided diagnosis of serrated colorectal lesions using non-magnified white-light endoscopic images. Int J Colorectal Dis 37, 1875–1884 (2022). https://doi.org/10.1007/s00384-022-04210-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04210-x