Abstract
Introduction
In metastasis research, modern microscopic techniques shed a new light on the mechanisms of metastatic tumor cell arrest in the microcirculation of potential metastasis target organs. In this study, we differentiated the contribution of mechanical cell arrest, determined as lumen occlusion of liver sinusoids by tumor cells, and specific cell adhesion mediated by integrins for the arrest of human colon cancer cells in rat livers.
Materials and methods
Using in vivo microscopy, the diameters of liver sinusoids of two different rat strains (CD, 250–300 g and RNU, 80–120 g) were determined. Cells (HT-29LMM) were intracardially injected, and the numbers of arrested cells and the rates of sinusoid occluding cells were determined.
Results
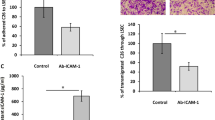
Mean sinusoid diameter in CD rats was 6.98 ± 1.42 µm compared to 5.14 ± 1.11 µm in RNU rats (p < 0.001). The numbers of arrested tumor cells and the rates of extravasated tumor cells did not differ between the two rat strains. Nevertheless, 5 and 30 min after cell injection, 35 ± 15% and 19 ± 8% of arrested cells, respectively, appeared lumen occluding in RNU rats and 9 ± 6% and 3 ± 3%, respectively, in CD rats (p < 0.05). Despite the higher rates of lumen occlusive cells in RNU rats, inhibition of beta-1 or beta-4 integrins significantly impaired cell arrest by 30–60% in both strains.
Discussion
In summary, these results demonstrate that lumen occlusion alone, as determined by in vivo microscopy, is insufficient to establish stable tumor cell arrest of colon carcinoma cells in metastatic target organs and does therefore not rule out the requirement of specific adhesive interactions for tumor cell arrest in the microcirculation.




Similar content being viewed by others
References
Morris M, Iacopetta B, Platell C (2007) Comparing survival outcomes for patients with colorectal cancer treated in public and private hospitals. Med J Aust 186:296–300
Weiss L (1992) Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 10:191–199
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer 2:563–572
Mook OR, Van Marle J, Vreeling-Sindelarova H, Jonges R, Frederiks WM, Van Noorden CJ (2003) Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology 38:295–304
Steinbauer M, Guba M, Cernaianu G et al (2003) GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term tumor development studies in immunocompetent mice. Clin Exp Metastasis 20:135–141
Ding L, Sunamura M, Kodama T et al (2001) In vivo evaluation of the early events associated with liver metastasis of circulating cancer cells. Br J Cancer 85:431–438
Mizuno N, Kato Y, Shirota K et al (1998) Mechanism of initial distribution of blood-borne colon carcinoma cells in the liver. J Hepatol 28:878–885
Ewing J (1928) Neoplastic diseases, 6th edn. Saunders, Philadelphia
Glinskii OV, Huxley VH, Glinsky GV et al (2005) Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cel arrest in distant organs. Neoplasia 5:522–527
Wang H, Fu W, Im JH et al (2004) Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J Cell Biol 164:935–941
Enns A, Gassmann P, Schlüter K et al (2004) Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg 8:1049–1059
Orr FW, Wang HH (2001) Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg Oncol Clin N Am 10:357–381
Schlüter K, Gassmann P, Enns A et al (2006) Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol 169:1064–1073
Korb T, Schlüter K, Enns A et al (2004) Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res 299:236–247
Glinsky VV, Glinsky GV, Glinskii OV et al (2003) Intravascular metastatic cancer cell aggregation at the sites of primary attachment to the endothelium. Cancer Res 63:3805–3811
Haier J, Nicolson GL (2002) PTEN regulates tumor cell adhesion of colon carcinoma cells under dynamic conditions of fluid flow. Oncogene 21:1450–1460
Haier J, Korb T, Hotz B et al (2003) An intravital model to monitor steps of metastatic tumor cell adhesion within the hepatic microcirculation. J Gastrointest Surg 7:507–514
Paget S (1889) The distribution of secondary growth in cancer of the breast. Lancet 1:571–573
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
García-Olmo DC, García-Rivas M, García-Olmo D et al (2003) The site of injection of tumor cells in rats does not influence the subsequent distribution of metastases. Oncol Rep 10:903–907
Luzzi KJ, MacDonald IC, Schmidt EE et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873
Glinskii OV, Huxley VH, Turk JR et al (2003) Continuous real time ex vivo epifluorescent video microscopy for the study of metastatic cancer cell interactions with microvascular endothelium. Clin Exp Metastasis 20:451–458
Zipin A, Israeli-Amit M, Meshel T et al (2004) Tumor-microenvironment interactions: the fucose-generating FX enzyme controls adhesive properties of colorectal cancer cells. Cancer Res 64:6571–6578
Shimizu S, Yamada N, Sawada T et al (2000) Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes. Pathol Int 50:953–959
Vogel I, Shen Y, Soeth E et al (1998) A human carcinoma model in athymic rats reflecting solid and disseminated colorectal metastases. Langenbecks Arch Surg 383:466–473
Wang M, Vogel I, Kalthoff H (2003) Correlation between metastatic potential and variants from colorectal tumor cell line HT-29. World J Gastroenterol 9:2627–2631
Vekemans K, Braet F, Wisse E (2004) DiO-labeled CC531s colon carcinoma cells traverse the hepatic sinusoidal endothelium via the Fas/FasL pathway. J Gastrointest Surg 8:371–372
Acknowledgment
These data were also presented at the 124th Congress of the German Society of Surgery, May 1st to 5th 2007 at the ICM, Munich, Germany. The authors thank K. Hagen for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gassmann, P., Hemping-Bovenkerk, A., Mees, S.T. et al. Metastatic tumor cell arrest in the liver–lumen occlusion and specific adhesion are not exclusive. Int J Colorectal Dis 24, 851–858 (2009). https://doi.org/10.1007/s00384-009-0694-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0694-2