Abstract
Background
An impaired production of interleukin (IL)-12 and T cell interferon-γ (IFN-γ) of in vitro stimulated monocytes has been discussed as a pathogenic factor in Whipple’s disease (WD). It is unclear whether this defect of cellular immunity is translated to the humoral immune system and to serum correlates.
Methods
We analyzed the serum of 40 patients with Whipple’s disease in various degrees of disease activity by sandwich enzyme-linked immunosorbent assay for differences in cytokine and cell adhesion molecule concentrations compared with age- and sex-matched controls.
Results
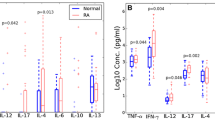
We observed a highly significant reduction of IL-12p40 levels (patients, 0.18±0.05 ng/ml (mean±SEM); controls, 3.19±0.39 ng/ml; p<0.01) in all stages of disease activity, whereas the concentration of IL-12p70 was comparable with controls. Furthermore, we observed a slight decrease in tumour necrosis factor α (TNF-α) concentrations in the serum of patients (patients, 6.36±0.90 pg/ml; controls, 10.5±1.23 pg/ml; p<0,05). The levels of other cytokines such as IFN-γ, IL-2, IL-13 and transforming growth factor β, as well as soluble cell adhesion molecules lymphocyte function-associated antigen 3 and intercellular adhesion molecule 1, were not significantly different compared with controls. Levels of immunoglobulin G2 (IgG2) measured in the serum of WD patients were below normal in 24 of 29 patients and were even below the 95% confidence interval in 10 patients.
Conclusion
Our data demonstrate a persistent defect of the cellular immune response with decreased serum concentrations of IL-12p40 and TNF-α and decreased IgG2 levels in a large group of WD patients. These data support as in vivo finding the results obtained in previous investigations with stimulated monocytes/lymphocytes. The isolated decrease in IL-12p40 may hint at possible defects in the IL-12/IFN-γ promoter system.



Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- ELISA:
-
enzyme-linked immunosorbent assay
- ICAM:
-
intercellular adhesion molecule
- ICSBP:
-
interferon consensus sequence binding protein
- IFN-γ:
-
interferon gamma
- IgG2:
-
immunoglobulin G2
- IL:
-
interleukin
- LFA:
-
lymphocyte function-associated antigen
- LPS:
-
lipopolysaccharide
- SD:
-
standard deviation
- TGF-β:
-
transforming growth factor beta
- TNF-α:
-
tumor necrosis factor alpha
- WD:
-
Whipple’s disease
References
Whipple GH (1907) A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp 18:382–391
Singer R (1998) Diagnosis and treatment of Whipple’s disease. Drugs 55:699–704
Dobbins WO III (1987) Whipple’s disease. Charles C. Thomas, Springfield, IL
Marth T (1996) Whipple-Erkrankung. In: Hahn EG, Riemann JF (eds) Klinische Gastroenterologie, 3rd edn. Thieme, Stuttgart, pp 947–951
Fenollar F, Raoult D (2001) Whipple’s disease. Minireview. Clin Diagn Lab Immunol 8:1–8
Marth T, Strober W (1996) Whipple’s disease. Semin Gastrointest Dis 7:41–48
Maiwald M, Relman DA (2001) Whipple’s disease and Tropheryma whippelii: secrets slowly revealed. Clin Infect Dis 32:457–463
Marth T, Raoult D (2003) Whipple’s disease. Lancet 361:239–246
Black-Schaffer B (1949) Tinctorial demonstration of a glycoprotein in Whipple’s disease. Proc Soc Exp Biol Med 72:225
Fredricks DN, Relman DA (2001) Localization of Tropheryma whippelii rRNA in tissues from patients with Whipple’s disease. J Infect Dis 183:1229–1237
Trier JS, Phelps PC, Eidelman S et al (1965) Whipple’s disease: light and electron microscopic correlation of jejunal mucosal histology with antibiotic treatment and clinical status. Gastroenterology 48:684–707
Wilson KH, Blitchington R, Frothingham R, Wilson JAP (1991) Phylogeny of the Whipple’s disease-associated bacterium. Lancet II:474
Raoult D, Birg ML, La Scola B, Fournier PE, Enea M, Lepidi H, Roux V, Piette J-C, Vandenesch F, Vital-Durand D, Marrie TJ (2000) Cultivation of the bacillus of Whipple’s disease. N Engl J Med 342:620–625
Raoult D, La Scola B, Lecocq P, Lepidi H, Fournier PE (2001) Culture and immunological detection of Tropheryma whippelii in the duodenum of a patient with Whipple’s disease. JAMA 285:1039–1043
La Scola B, Fenollar F, Fournier PE, Altwegg M, Mallet MN, Raoult D (2001) Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int J Syst Evol Microbiol 51:1471–1479
Liang Z, La Scola B, Raoult D (2002) Monoclonal antibodies to immunodominant epitope of Tropheryma whipplei. Clin Diagn Lab Immunol 9:156–159
Raoult D, Lepidi H, Harle JR (2001) Tropheryma whippelii circulating in blood monocytes. N Engl J Med 345:548
Relman DA, Schmidt TM, Mac Dermott RP, Falkow S (1992) Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 327:293–301
Feurle GE, Dörken B, Schöpf E, Lenhard V (1979) HLA-B27 and defects in the T cell system in Whipple’s disease. Eur J Clin Investig 9:385
Feurle GE (1985) Association of Whipple’s disease with HLA-B27. Lancet 8:1336
Paulley JW (1952) A case of Whipple’s disease (intestinal lipodystrophy). Gastroenterol 22:128
Maiwald M, Schuhmacher F, Ditton H-J, von Herbay A (1998) Environmental occurrence of the Whipple’s disease bacterium (Tropheryma whippelii). Appl Environ Microbiol 64:760–762
Bjerknes R, Ødegaard S, Bjerkvig R, Børkje B, Lærum OD (1988) Whipple’s disease: demonstration of a persisting monocyte and macrophage dysfunction. Scand J Gastroenterol 23:611–619
Marth T, Roux M, von Herbay A, Meuer SC, Feurle GE (1994) Persistent reduction of complement receptor 3 α-chain expressing mononuclear blood cells and transient inhibitory serum factors in Whipple’s disease. Clin Immunol Immunopathol 72:217–226
Marth T, Neurath M, Cuccherini BA, Strober W (1997) Defects of monocyte interleukin-12 production and humoral immunity in Whipple’s disease. Gastroenterology 113:442–448
Marth T, Kleen N, Stallmach A, Ring S, Aziz S, Schmidt C, Strober W, Zeitz M, Schneider T (2002) Dysregulated peripheral and mucosal Th1/Th2 response in Whipple’s disease. Gastroenterology 123:1468–1477
Schoedon G, Goldenberger D, Forrer R, Gunz A, Dutly F, Hoechli M, Altwegg M, Schaffner A (1997) Deactivation of macrophages with Interleukin-4 is the key to the isolation of Tropheryma whippelii. J Infect Dis 176:672–677
Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski FJ, Gately MK, Rude E (1995) Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol 25:823–829
Janeway CA, Travers P, Walport M, Shlomchik M (2001) Immunobiology: the immune system in health and disease, 5th edn. Garland, London
Hoffmann JC, Dengler TJ, Knolle PA, Albert-Wolf M, Roux M, Wallich R, Meuer SC (1993) A soluble form of the adhesion receptor CD58 (LFA-3) is present in human body fluids. Eur J Immunol 23:3003–3010
Trinchieri G (1994) Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008–4027
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146
Trinchieri G (1997) Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ). Curr Opin Immunol 9:17
Hayes MP, Wang J, Norcross MA (1995) Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood 86:646
Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G (1996) The interleukin 12p40 gene promoter is primed by interferon-γ in monocytic cells. J Exp Med 183:147
Wang IM, Contursi C, Masumi A, Ma X, Trinchieri G, Ozato K (2000) An IFN-γ-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12p40 expression in macrophages. J Immunol 165:271–279
Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL (1998) Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432–1435
Picard C, Fieschi C, Altare F, Al-Jumaah S, Al-Hajjar S, Feinberg J, Dupuis S, Soudais C, Al-Mohsen IZ, Genin E, Lammas D, Kumararatne DS, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah LB, Sinniah R, Loubser M, Okamoto E, Al-Ghonaium A, Tufenkeji H, Abel L, Casanova JL (2002) Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet 70:336–348
Acknowledgements
We would like to acknowledge Prof. Dr. Feurle, Neuwied, Germany, for the provision of serum samples of some patients of group 1 (n=3), which have already been included in a therapy study, Studie zur Initialtherapie des Morbus Whipple (SIMW) of WD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalt, A., Schneider, T., Ring, S. et al. Decreased levels of interleukin-12p40 in the serum of patients with Whipple’s disease. Int J Colorectal Dis 21, 114–120 (2006). https://doi.org/10.1007/s00384-005-0778-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-005-0778-6