Abstract
Purpose
Intestinal neuronal dysplasia (IND) is a congenital anomaly affecting gastrointestinal neural innervation, but the pathogenesis remains unclear. The homozygous Ncx/Hox11L.1 knockout (Ncx−/−) mice exhibit megacolon and enteric ganglia anomalies, resembling IND phenotypes. Sox10-Venus transgenic mouse were used to visualize enteric neural crest cells in real time. This study aims to establish a novel mouse model of Sox10-Venus+/Ncx−/− mouse to study the pathogenesis of IND.
Methods
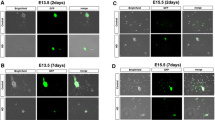
Sox10-Venus+/Ncx−/− (Ncx−/−) (n = 8) mice and Sox10-Venus+/Ncx+/+ controls (control) (n = 8) were euthanized at 4–5 weeks old, and excised intestines were examined with fluorescence microscopy. Immunohistochemistry was performed on tissue sections with neural marker Tuj1.
Results
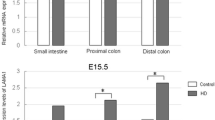
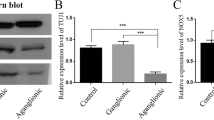
Ncx−/− mice exhibited dilated cecum and small intestine. Body weight of Ncx−/− mice was lower with higher ratio of small intestine length relative to body weight. The neural network (Sox10-Venus) was observed along the intestine wall in Ncx−/− and control mice without staining. Ectopic and increased expression of Tuj1 was observed in both small intestine and proximal colon of Ncx−/− mice.
Conclusion
This study has established a reliable animal model that exhibits characteristics similar to patients with IND. This novel mouse model can allow the easy visualization of ENS in a time- and cost-effective way to study the pathogenesis of IND.



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kapur RP, Reyes-Mugica M (2019) Intestinal neuronal dysplasia type B: an updated review of a problematic diagnosis. Arch Pathol Lab Med 143:235–243. https://doi.org/10.5858/arpa.2017-0524-RA
Yamataka A, Hatano M, Kobayashi H, Wang K, Miyahara K, Sueyoshi N et al (2001) Intestinal neuronal dysplasia-like pathology in Ncx/Hox11L.1 gene-deficient mice. J Pediatr Surg 36:1293–1296. https://doi.org/10.1053/jpsu.2001.25797
Meier-Ruge W (1971) Casuistic of colon disorder with symptoms of Hirschsprung’s disease (author’s transl). Verh Dtsch Ges Pathol 55:506–510
Liu W, Zhou T, Tian J, Yu X, Ren C, Cao Z et al (2022) Role of GDNF, GFRα1 and GFAP in a bifidobacterium-intervention induced mouse model of intestinal neuronal dysplasia. Front Pediatr. https://doi.org/10.3389/fped.2021.795678
Wang D, Gao N, Zhou T, Zhang Q, Wang J, Li A (2020) Effect of Neuroligin1 and Neurexin1 on the colonic motility in a mouse model of neuronal intestinal dysplasia. Gastroenterol Res Pract 2020:1–9. https://doi.org/10.1155/2020/9818652
Hatano M, Aoki T, Dezawa M, Yusa S, Iitsuka Y, Koseki H et al (1997) A novel pathogenesis of megacolon in Ncx/Hox11L.1 deficient mice. J Clin Investig 100:795–801. https://doi.org/10.1172/JCI119593
Shirasawa S, Yunker AMR, Roth KA, Brown GA, Horning S, Korsmeyer SJ (1997) Enx (Hox11L1)-deficient mice develop myenteric neuronal hyperplasia and megacolon. Nat Med 3:646–650. https://doi.org/10.1038/nm0697-646
Wen X-Y, Tang S, Breitman ML (1994) Genetic mapping of two mouse homeobox genes Tlx-1 and Tlx-2 to murine chromosomes 19 and 6. Genomics 24:388–390. https://doi.org/10.1006/geno.1994.1634
Dear TN, Sanchez-Garcia I, Rabbitts TH (1993) The HOX11 gene encodes a DNA-binding nuclear transcription factor belonging to a distinct family of homeobox genes. Proc Natl Acad Sci 90:4431–4435. https://doi.org/10.1073/pnas.90.10.4431
Hatano M, Iitsuka Y, Yamamoto H, Dezawa M, Yusa S, Kohno Y et al (1997) Ncx, a Hox11 related gene, is expressed in a variety of tissues derived from neural crest cells. Anat Embryol (Berl) 195:419–425. https://doi.org/10.1007/s004290050061
Kelsh RN (2006) Sorting outSox10 functions in neural crest development. BioEssays 28:788–798. https://doi.org/10.1002/bies.20445
Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V (2006) Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development 133:2075–2086. https://doi.org/10.1242/dev.02375
Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V (2003) Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130:6387–6400. https://doi.org/10.1242/dev.00857
Shibata S, Yasuda A, Renault-Mihara F, Suyama S, Katoh H, Inoue T et al (2010) Sox10- Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3:31. https://doi.org/10.1186/1756-6606-3-31
Miyahara K, Kato Y, Koga H, Lane GJ, Inoue T, Akazawa C et al (2010) Abnormal enteric innervation identified without histopathologic staining in aganglionic colorectum from a mouse model of Hirschsprung’s disease. J Pediatr Surg 45:2403–2407. https://doi.org/10.1016/j.jpedsurg.2010.08.039
Fujiwara N, Miyahara K, Nakazawa-Tanaka N, Akazawa C, Yamataka A (2022) In vitro investigation of the differentiation of enteric neural crest-derived cells following transplantation of aganglionic gut in a mouse model. Pediatr Surg Int 38:755–759. https://doi.org/10.1007/s00383-022-05105-2
Granero Cendón R, Millán López A, Moya Jiménez MJ, López Alonso M (2007) De Agustín Asensio JC [Intestinal neuronal dysplasia: association with digestive malformations]. Cir Pediatr 20:166–168
Puri P, Rolle U (2004) Variant Hirschsprung’s disease. Semin Pediatr Surg 13:293–299. https://doi.org/10.1053/j.sempedsurg.2004.10.017
Puri P, Gosemann J-H (2012) Variants of Hirschsprung disease. Semin Pediatr Surg 21:310–318. https://doi.org/10.1053/j.sempedsurg.2012.07.005
Fadda B, Maier W, Meier-Ruge W, Schärli A, Daum R (1983) Neuronale intestinale DysplasieEine kritische 10-Jahres-Analyse klinischer und bioptischer Diagnostik. Eur J Pediatr Surg 38:305–311. https://doi.org/10.1055/s-2008-1059994
Puri P (1997) Variant Hirschsprung’s disease. J Pediatr Surg 32:149–157. https://doi.org/10.1016/S0022-3468(97)90170-6
Gath R, Goessling A, Keller K-M, Koletzko S, Coerdt W, Müntefering H et al (2001) Analysis of the RET, GDNF, EDN3, and EDNRB genes in patients with intestinal neuronal dysplasia and Hirschsprung disease. Gut 48:671–675. https://doi.org/10.1136/gut.48.5.671
Sánchez-Mejías A, Fernández RM, Antiñolo G, Borrego S (2010) A new experimental approach is required in the molecular analysis of intestinal neuronal dysplasia type B patients. Exp Ther Med 1:999–1003. https://doi.org/10.3892/etm.2010.140
Borghini S, Di DM, Prato AP, Lerone M, Martucciello G, Jasonni V et al (2009) Search for pathogenetic variants of the SPRY2 gene in intestinal innervation defects. Intern Med J 39:335–337. https://doi.org/10.1111/j.1445-5994.2009.01907.x
Fava M, Borghini S, Cinti R, Cusano R, Seri M, Lerone M et al (2002) HOX11L1: a promoter study to evaluate possible expression defects in intestinal motility disorders. Int J Mol Med 10:101–106
Costa M (2000) Evaluation of the HOX11L1 gene as a candidate for congenital disorders of intestinal innervation. J Med Genet 37:9e–99. https://doi.org/10.1136/jmg.37.7.e9
Sacher P, Briner J, Hanimann B (1993) Is neuronal intestinal dysplasia (NID) a primary disease or a secondary phenomenon? Eur J Pediatr Surg 3:228–230. https://doi.org/10.1055/s-2008-1063549
von Boyen GBT (2002) Abnormalities of the enteric nervous system in heterozygous endothelin B receptor deficient (spotting lethal) rats resembling intestinal neuronal dysplasia. Gut 51:414–419. https://doi.org/10.1136/gut.51.3.414
Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R et al (2005) Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci 8:855–857. https://doi.org/10.1038/nn1485
Yanai T, Kobayashi H, Yamataka A, Lane GJ, Miyano T, Hayakawa T et al (2004) Acetylcholine-related bowel dysmotility in homozygous mutant NCX/HOX11L.1-deficient (NCX−/−) mice—evidence that acetylcholine is implicated in causing intestinal neuronal dysplasia. J Pediatr Surg 39:927–930. https://doi.org/10.1016/j.jpedsurg.2004.02.004
Young HM, Bergner AJ, Müller T (2003) Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol 456:1–11. https://doi.org/10.1002/cne.10448
Liu Y-R, Ba F, Cheng L-J, Li X, Zhang S-W, Zhang S-C (2019) Efficacy of Sox10 promoter methylation in the diagnosis of intestinal neuronal dysplasia from the peripheral blood. Clin Transl Gastroenterol 10:e00093. https://doi.org/10.14309/ctg.0000000000000093
Acknowledgements
We thank Mr. Fumio Kanai for support for the animals in the Laboratory of Genome Research, Research Institute for Diseases of Old Age, Juntendo University Graduate School of Medicine and Ms. Mirei Takahashi for assistance in the experiments.
Funding
This work was supported by JSPS KAKENHI Grant Number (22K08721).
Author information
Authors and Affiliations
Contributions
NF performed animal experiments and the analysis of the experimental results, also wrote the whole manuscript. KM performed some of the animal experiments. NN-T and DL supervised some of the animal experiments. AP, AY, CA and MH provided advice and supervision. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujiwara, N., Miyahara, K., Lee, D. et al. A novel mouse model of intestinal neuronal dysplasia: visualization of the enteric nervous system. Pediatr Surg Int 39, 298 (2023). https://doi.org/10.1007/s00383-023-05585-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05585-w