Abstract
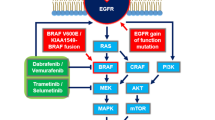
Since the discovery of the association between BRAF mutations and fusions in the development of childhood low-grade gliomas and the subsequent recognition that most childhood low-grade glial and glioneuronal tumors have aberrant signaling through the RAS/RAF/MAP kinase pathway, there has been a dramatic change in how these tumors are conceptualized. Many of the fusions and mutations present in these tumors are associated with molecular targets, which have agents in development or already in clinical use. Various agents, including MEK inhibitors, BRAF inhibitors, MTOR inhibitors and, in small subsets of patients NTRK inhibitors, have been used successfully to treat children with recurrent disease, after failure of conventional approaches such as surgery or chemotherapy. The relative benefits of chemotherapy as compared to molecular-targeted therapy for children with newly diagnosed gliomas and neuroglial tumors are under study. Already the combination of an MEK inhibitor and a BRAF inhibitor has been shown superior to conventional chemotherapy (carboplatin and vincristine) in newly diagnosed children with BRAF-V600E mutated low-grade gliomas and neuroglial tumors. However, the long-term effects of such molecular-targeted treatment are unknown. The potential use of molecular-targeted therapy in early treatment has made it mandatory that the molecular make-up of the majority of low-grade glial and glioneuronal tumors is known before initiation of therapy. The primary exception to this rule is in children with neurofibromatosis type 1 who, by definition, have NF1 loss; however, even in this population, gliomas arising in late childhood and adolescence or those not responding to conventional treatment may be candidates for biopsy, especially before entry on molecular-targeted therapy trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
No datasets were generated or analysed during the current study.
References
Packer RJ, Pfister S, Bouffet E et al (2017) Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol 19(6):750–761
Packer RJ, Iavarone A, Jones DTW et al (2020) Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro Oncol 22(6):773–784
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Pfister S, Janzarik WG, Remke M et al (2008) BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest 118(5):1739–1749
Jones DT, Kocialkowski S, Liu L et al (2008) Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res 68(21):8673–8677
Jones DT, Hutter B, Jager N et al (2013) Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 45(8):927–932
Jones DTW, Kieran MW, Bouffet E et al (2018) Pediatric low-grade gliomas: next biologically driven steps. Neuro-Oncology 20(2):160–173
Ryall S, Tabori U, Hawkins C (2020) Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun 8(1):30
Ji RR, Gereau RW, Malcangio M et al (2009) MAP kinase and pain. Brain Res Rev 60(1):135–148
Sweatt JD (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76(1):1–10
Lassaletta A, Zapotocky M, Mistry M et al (2017) Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol 35(25):2934–2941
Becker AP, Scapulatempo-Neto C, Carloni AC et al (2015) KIAA1549: BRAF gene fusion and FGFR1 hotspot mutations are prognostic factors in pilocytic astrocytomas. J Neuropathol Exp Neurol 74(7):743–754
Kim M, Lee KR, Choe G et al (2023) Diffuse leptomeningeal glioneuronal tumor with FGFR1 mutation in a 29-year-old male. J Korean Soc Radiol 84(4):970–976
Meredith DM, Cooley LD, Dubuc A et al (2023) ROS1 alterations as a potential driver of gliomas in infant, pediatric, and adult patients. Mod Pathol 36(11):100294
de Blank PMK, Fisher MJ, Liu GT et al (2017) Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuro-ophthalmol 37:S23–S32
D'Angelo F, Ceccarelli M (2019) Tala et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 25(1):176–187
Fisher MJ, Jones DTW, Li Y et al (2021) Integrated molecular and clinical analysis of low-grade gliomas in children with neurofibromatosis type 1 (NF1). Acta Neuropathol 141(4):605–617
Lucas CG, Sloan EA, Gupta R et al (2022) Multiplatform molecular analyses refine classification of gliomas arising in patients with neurofibromatosis type 1. Acta Neuropathol 144(4):747–765
Bandopadhayay P, Ramkissoon LA, Jain P et al (2016) MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet 48(3):273–282
Qaddoumi I, Orisme W, Wen J et al (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131(6):833–845
Moreira DC, Qaddoumi I, Spiller S et al (2024) Comprehensive analysis of MYB/MYBL1-altered pediatric-type diffuse low-grade glioma. Neuro Oncol. https://doi.org/10.1093/neuonc/noae048
Northcott PA, Pfister SM, Jones DT (2015) Next-generation (epi)genetic drivers of childhood brain tumours and the outlook for targeted therapies. Lancet Oncol 16(6):e293–e302
Braunstein S, Raleigh D, Bindra R et al (2017) Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol 134(3):541–549
Ryall S, Krishnatry R, Arnoldo A et al (2016) Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun 4(1):93
Jones DT, Gronych J, Lichter P et al (2012) MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci 69(11):1799–1811
Banerjee A, Jakacki RI, Onar-Thomas A et al (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncology 19(8):1135–1144
Fangusaro J, Onar-Thomas A, Young Poussaint T et al (2019) Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20(7):1011–1022
Fangusaro J, Onar-Thomas A, Poussaint TY et al (2021) A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium study. Neuro Oncol 23(10):1777–1788
U.S. Prescribing Information, Selumetinib, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/213756s004lbl.pdf. Accessed 23 May 2024
Perreault S, Larouche V, Tabori U et al (2019) A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 19(1):1250
Selt F, van Tilburg CM, Bison B et al (2020) Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol 149(3):499–510
Manoharan N, Choi J, Chordas C et al (2020) Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neurooncol 149(2):253–262
Paul MR, Pehlivan KC, Milburn M et al (2020) Trametinib-based treatment of pediatric CNS tumors: a single institutional experience. J Pediatr Hematol Oncol 42(8):e730–e737
Kondyli M, Larouche V, Saint-Martin C et al (2018) Trametinib for progressive pediatric low-grade gliomas. J Neurooncol 140(2):435–444
U.S. Prescribing Information, Binimetinib, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/210498s009lbl.pdf. Accessed 23 May 2024
U.S. Prescribing Information, Cobimetinib, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/206192s006lbl.pdf. Accessed 23 May 2024
Trippett T, Toledano H, Campbell Hewson Q et al (2022) Cobimetinib in pediatric and young adult patients with relapsed or refractory solid tumors (iMATRIX-cobi): a multicenter, phase I/II study. Target Oncol 17(3):283–293
Robison N, Pauly J, Malvar J et al (2022) LTBK-04. LATE BREAKING ABSTRACT: MEK162 (binimetinib) in children with progressive or recurrent low-grade glioma: a multi-institutional phase II and target validation study. Neuro-Oncology 24(Supplement_1):i191–i192
Bouffet E, Hansford JR, Garre ML et al (2023) Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N Engl J Med 389(12):1108–1120
U.S. Prescribing Information, Trametinib, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/204114s025lbl.pdf. Accessed 23 May 2024
Odogwu L, Mathieu L, Blumenthal G et al (2018) FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist 23(6):740–745
Hargrave DR, Bouffet E, Tabori U et al (2019) Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res 25(24):7303–7311
Bouffet E, Geoerger B, Moertel C et al (2023) Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J Clin Oncol 41(3):664–674
Desai AV, Robinson GW, Gauvain K et al (2022) Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1, or ALK aberrations (STARTRK-NG). Neuro Oncol 24(10):1776–1789
Banerjee S, Crouse NR, Emnett RJ et al (2011) Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci U S A 108(38):15996–16001
Nicolaides T, Nazemi KJ, Crawford J et al (2020) Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget 11(21):1942–1952
Sievert AJ, Lang SS, Boucher KL et al (2013) Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A 110(15):5957–5962
Karajannis MA, Legault G, Fisher MJ et al (2014) Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol 16(10):1408–1416
Sun Y, Alberta JA, Pilarz C et al (2017) A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol 19(6):774–785
Hutt-Cabezas M, Karajannis MA, Zagzag D et al (2013) Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol 15(12):1604–1614
U.S. Prescribing Information, Everolimus, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022334s6lbl.pdf. Accessed 23 May 2024
Ullrich NJ, Prabhu SP, Reddy AT et al (2020) A phase II study of continuous oral mTOR inhibitor everolimus for recurrent, radiographic-progressive neurofibromatosis type 1-associated pediatric low-grade glioma: a Neurofibromatosis Clinical Trials Consortium study. Neuro Oncol 22(10):1527–1535
Wright KD, Yao X, London WB et al (2021) A POETIC Phase II study of continuous oral everolimus in recurrent, radiographically progressive pediatric low-grade glioma. Pediatr Blood Cancer 68(2):e28787
Wright K, Krzykwa E, Greenspan L et al (2020) Ctni-19. Phase I trial of day101 in pediatric patients with radiographically recurrent or progressive low-grade glioma (Lgg). Neuro-Oncology 22(Supplement_2):ii46
Kilburn LB, Khuong-Quang DA, Hansford JR et al (2024) The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial. Nat Med 30(1):207–217
van Tilburg CM, Kilburn LB, Perreault S et al (2024) LOGGIC/FIREFLY-2: a phase 3, randomized trial of tovorafenib vs. chemotherapy in pediatric and young adult patients with newly diagnosed low-grade glioma harboring an activating RAF alteration. BMC Cancer 24(1):147
Lassman AB, Sepulveda-Sanchez JM, Cloughesy TF et al (2022) Infigratinib in patients with recurrent gliomas and FGFR alterations: a multicenter phase II study. Clin Cancer Res 28(11):2270–2277
Doz F, van Tilburg CM, Geoerger B et al (2022) Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol 24(6):997–1007
Haas-Kogan DA, Aboian MS, Minturn JE et al (2024) Everolimus for children with recurrent or progressive low-grade glioma: results from the phase II PNOC001 trial. J Clin Oncol 42(4):441–451
Packer RJ, Jakacki R, Horn M et al (2009) Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 52(7):791–795
Gorsi HS, Khanna PC, Tumblin M et al (2018) Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: a single institutional experience. Pediatr Blood Cancer 65(9):e27234
Gururangan S, Fangusaro J, Poussaint TY et al (2014) Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas - a Pediatric Brain Tumor Consortium study. Neuro-Oncology 16(2):310–317
Green K, Panagopoulou P, D'Arco F et al (2022) A nationwide evaluation of bevacizumab-based treatments in paediatric low-grade glioma in the UK: safety. efficacy, visual morbidity and outcomes. Neuro Oncol 25(4):774–785
de Marcellus C, Tauziede-Espariat A, Cuinet A et al (2022) The role of irinotecan-bevacizumab as rescue regimen in children with low-grade gliomas: a retrospective nationwide study in 72 patients. J Neurooncol 157(2):355–364
Siegel BI, Nelson D, Peragallo JH et al (2023) Visual outcomes after bevacizumab-based therapy for optic pathway glioma. Pediatr Blood Cancer 70(12):e30668
Heidary G, Fisher MJ, Liu GT et al (2020) Visual field outcomes in children treated for neurofibromatosis type 1-associated optic pathway gliomas: a multicenter retrospective study. J AAPOS 6:349.e341-349.e345
Bennebroek CAM, van Zwol J, Porro GL et al (2022) Impact of bevacizumab on visual function, tumor size, and toxicity in pediatric progressive optic pathway glioma: a retrospective nationwide multicentre study. Cancers (Basel) 14(24):6087
Hwang EI, Jakacki RI, Fisher MJ et al (2013) Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer 60(5):776–782
Mulcahy Levy JM, Zahedi S, Griesinger AM et al (2017) Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife 6:e19671
Touat M, Li YY, Boynton AN et al (2020) Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580(7804):517–523
Burton EM, Amaria RN, Glitza IC et al (2021) Phase II Study of TRIplet combination Nivolumab (N) with Dabrafenib (D) and Trametinib (T) (TRIDeNT) in patients (pts) with PD-1 naïve or refractory BRAF-mutated metastatic melanoma (MM) with or without active brain metastases. J Clin Oncol 39(15_suppl):9520–9520
Urban H, Steidl E, Hattingen E et al (2022) Immune checkpoint inhibitor-induced cerebral pseudoprogression: patterns and categorization. Front Immunol 12:798811
Hardin EC, Schmid S, Sommerkamp A et al (2023) LOGGIC Core BioClinical Data Bank: Added clinical value of RNA-Seq in an international molecular diagnostic registry for pediatric low-grade glioma patients. Neuro Oncol 25(11):2087–2097
Bitterman DS, MacDonald SM, Yock TI et al (2019) Revisiting the role of radiation therapy for pediatric low-grade glioma. J Clin Oncol 37(35):3335–3339
Fangusaro J, Avery RA, Fisher MJ et al (2024) Considering functional outcomes as efficacy endpoints in pediatric low-grade glioma clinical trials: an FDA educational symposium. Clin Cancer Res 30(11):2303–2308
Wan MJ, Ullrich NJ, Manley PE et al (2016) Long-term visual outcomes of optic pathway gliomas in pediatric patients without neurofibromatosis type 1. J Neurooncol 129(1):173–178
Jacob K, Quang-Khuong DA, Jones DT et al (2011) Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res 17(14):4650–4660
Acknowledgements
We would like to thank the Gilbert Family Neurofibromatosis Institute of Children’s National Hospital and the many donors of the Brain Tumor Institute of Children’s National Hospital for their support.
Author information
Authors and Affiliations
Contributions
All authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siegel, B.I., Duke, E.S., Kilburn, L.B. et al. Molecular-targeted therapy for childhood low-grade glial and glioneuronal tumors. Childs Nerv Syst 40, 3251–3262 (2024). https://doi.org/10.1007/s00381-024-06486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-024-06486-6