Abstract
Aim
We aimed to describe the experience of a large single-center cohort for the clinical, radiological, and genetic characteristics, as well as to determine the efficacy of different anti-epileptic strategies in children and adults with tuberous sclerosis complex (TSC).
Methods
We carried out a historical cohort study on 91 TSC patients treated in a single center between 2008 and 2018.
Results
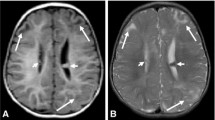
Our cohort comprised 46 males and 45 females, with a median age of 15.6 years at the last follow-up. Mean follow-up time was 2.5 ± 0.75–5.5 years (range 0–9.5 years). Of those tested, a disease-causing mutation was identified in 90% of patients, 53% in TSC2, and 37% in TSC1. Epilepsy prevalence was similar among TSC1 and TSC2 mutated patients. The most common radiological finding were cortical tubers in 95% of patients, while subependymal giant cell astrocytoma (SEGA) were detected in 36% of patients. Notably, infantile spasms (IS) were diagnosed in 29%, with SEGA representing the only finding significantly different in prevalence between those with and without IS (62% vs. 28%, respectively, p = 0.009). Lastly, we did not find any difference in efficacy between three anti-epileptic treatments: Vagus nerve stimulation (VNS), CBD-based products, and the ketogenic diet, all showing approximately 30%–40% response rates.
Significance
Altogether, we provide a comprehensive description of our experience in treating TSC, which could serve to expand current knowledge of the disease and its treatments.

Similar content being viewed by others
References
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355(13):1345–1356. https://doi.org/10.1056/NEJMra055323
Lam HC, Nijmeh J, Henske EP (2017) New developments in the genetics and pathogenesis of tumours in tuberous sclerosis complex. J Pathol 241(2):219–225. https://doi.org/10.1002/path.4827
Roach ES (2013) Are diagnostic criteria for tuberous sclerosis still relevant? Pediatr Neurol 49(4):223–224. https://doi.org/10.1016/j.pediatrneurol.2013.08.003
Northrup H, Krueger DA, Roberds S et al (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49(4):243–254
Yates JR, Maclean C, Higgins JN et al (2011) The Tuberous Sclerosis 2000 Study: presentation, initial assessments and implications for diagnosis and management. Arch Dis Child 96(11):1020–1025. https://doi.org/10.1136/adc.2011.211995
Goh S, Butler W, Thiele EAJN (2004) Subependymal giant cell tumors in tuberous sclerosis complex. Neurology 63(8):1457–1461
Jansen F, Vincken K, Algra A et al (2008) Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 70(12):916–923
Pirson YJNDT (2013) Tuberous sclerosis complex-associated kidney angiomyolipoma: from contemplation to action. Nephrol Dial Transplant 28(7):1680–1685
Pleniceanu O, Omer D, Azaria E, Harari-Steinberg O, Dekel BJK (2018) mTORC1 inhibition is an effective treatment for sporadic renal angiomyolipoma. Kidney Int Rep 3(1):155–159
Zöllner JP, Franz DN, Hertzberg C et al (2020) A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J Rare Dis 15(1):23
Lin S, Zeng JB, Zhao GX et al (2019) Tuberous sclerosis complex in chinese patients: phenotypic analysis and mutational screening of TSC1/TSC2 genes. Seizure 71:322–327
Reyna-Fabián ME, Hernández-Martínez NL, Alcántara-Ortigoza MA et al (2020) First comprehensive TSC1/TSC2 mutational analysis in Mexican patients with Tuberous Sclerosis Complex reveals numerous novel pathogenic variants. Sci Rep 10(1):1–14
Rosengren T, Nanhoe S, de Almeida LGD et al (2020) Mutational analysis of TSC1 and TSC2 in Danish patients with tuberous sclerosis complex. Sci Rep 10(1):1–9
Au KS, Williams AT, Roach ES et al (2007) Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 9(2):88–100
Dabora SL, Jozwiak S, Franz DN et al (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68(1):64–80
Jones AC, Shyamsundar MM, Thomas MW et al (1999) Comprehensive mutation analysis of TSC1 and TSC2—and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 64(5):1305–1315
Nellist M, Brouwer RW, Kockx CE et al (2015) Targeted Next Generation Sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Med Genet 16(1):1–11
Tyburczy ME, Dies KA, Glass J et al (2015) Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with no mutation identified by conventional testing. PLoS Genet 11(11):e1005637
Thiele EA (2010) Managing and understanding epilepsy in tuberous sclerosis complex. Epilepsia 51(Suppl 1):90–91. https://doi.org/10.1111/j.1528-1167.2009.02458.x
Kingswood JC, Bruzzi P, Curatolo P et al (2014) TOSCA–first international registry to address knowledge gaps in the natural history and management of tuberous sclerosis complex. Orphanet J Rare Dis 9(1):1–9
Lennert B, Farrelly E, Sacco P, Pira G, Frost M (2013) Resource utilization in children with tuberous sclerosis complex and associated seizures: a retrospective chart review study. J Child Neurol 28(4):461–469
Chan DL, Calder T, Lawson JA, Mowat D, Kennedy SE (2018) The natural history of subependymal giant cell astrocytomas in tuberous sclerosis complex: a review. Rev Neurosci 29(3):295–301
Adriaensen M, Schaefer-Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M (2009) Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol 16(6):691–696
Curatolo P, Seri S, Verdecchia M, Bombardieri R (2001) Infantile spasms in tuberous sclerosis complex. Brain Dev 23(7):502–507
Kingswood JC, d’Augères GB, Belousova E et al (2017) TuberOus SClerosis registry to increase disease Awareness (TOSCA)–baseline data on 2093 patients. Orphanet J Rare Dis 12(1):1–13
Kingswood C, Bolton P, Crawford P et al (2016) The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: A retrospective cohort study in the Clinical Practice Research Datalink (CPRD). Eur J Paediatr Neurol 20(2):296–308
Teng JM, Cowen EW, Wataya-Kaneda M et al (2014) Dermatologic and dental aspects of the 2012 international tuberous sclerosis complex consensus statements. JAMA Dermatol 150(10):1095–1101
Elliott RE, Carlson C, Kalhorn SP et al (2009) Refractory epilepsy in tuberous sclerosis: vagus nerve stimulation with or without subsequent resective surgery. Epilepsy Behav 16(3):454–460
Major P, Thiele EA (2008) Vagus nerve stimulation for intractable epilepsy in tuberous sclerosis complex. Epilepsy & behavior : E&B 13(2):357–360. https://doi.org/10.1016/j.yebeh.2008.04.001
Tzadok M, Harush A, Nissenkorn A, Zauberman Y, Feldman Z, Ben-Zeev BJS (2019) Clinical outcomes of closed-loop vagal nerve stimulation in patients with refractory epilepsy. Seizure 71:140–144
Youn SE, Park S, Kim SH, Lee JS, Kim HD, Kang HC (2020) Long-term outcomes of ketogenic diet in patients with tuberous sclerosis complex-derived epilepsy. Epilepsy Res 164:106348
Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA (2006) Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol 21(3):193–198
Lefevre F, Aronson N (2000) Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics 105(4):E46. https://doi.org/10.1542/peds.105.4.e46
Martin K, Jackson CF, Levy RG, Cooper PN (2016) Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001903.pub3
Hess EJ, Moody KA, Geffrey AL et al (2016) Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 57(10):1617–1624
Kavčič A, Kajdič N, Rener-Primec Z, Krajnc N, Žgur T (2019) Efficacy and tolerability of vagus nerve stimulation therapy (VNS) in Slovenian epilepsy patients: Younger age and shorter duration of epilepsy might result in better outcome. Acta Clin Croat 58(2):255–264
Englot DJ, Chang EF, Auguste KIJNC (2011) Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurg Clin N Am 22(4):443–448
Pleniceanu O, Shukrun R, Omer D et al (2017) Peroxisome proliferator-activated receptor gamma (PPARγ) is central to the initiation and propagation of human angiomyolipoma, suggesting its potential as a therapeutic target. EMBO Mol Med 9(4):508–530
Valvezan AJ, McNamara MC, Miller SK et al (2020) IMPDH inhibitors for antitumor therapy in tuberous sclerosis complex. JCI Insight. https://doi.org/10.1172/jci.insight.135071
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that there are no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shlomovitz, O., Ben-Zeev, B., Pleniceanu, O. et al. An Israeli tuberous sclerosis cohort: the efficacy of different anti-epileptic strategies. Childs Nerv Syst 37, 3827–3833 (2021). https://doi.org/10.1007/s00381-021-05348-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05348-9