Abstract
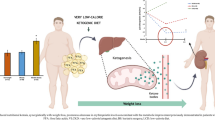
Results of experimental studies have shown that β-aminoisobutyric acid (BAIBA), an exercise-induced myokine-like molecule, is an endogenous negative regulator of fat mass in mice, but it remains unclear whether that is the case in humans, though an enhanced BAIBA concentration in patients receiving sodium–glucose cotransporter 2 inhibitors was found in our recent study. The objective of this study was to analyze the determinants of circulating BAIBA concentration in humans, with focus on the possible link between circulating BAIBA and body composition including fat mass. Data for 188 consecutive patients with heart failure (HF, 64 ± 13 years; 70% male) who received a dual energy X ray absorptiometry (DEXA) scan for assessment of body composition including fat mass index (FMI) and appendicular skeletal muscle mass index (ASMI) were used in this study. Plasma BAIBA concentration in a fasting state after stabilization of HF was determined using ultraperformance liquid chromatography. Plasma BAIBA was detected in 66% of the patients. In simple linear regression analyses of data from patients in whom plasma BAIBA was detected, plasma BAIBA concentration was positively correlated with uric acid and was negatively correlated with body mass index (BMI), estimated glomerular filtration rate (eGFR), FMI, and % body fat. There were no correlations between plasma BAIBA concentration and indexes of muscle mass and bone mass. The results of multiple linear regression analyses showed that FMI and % body fat in addition to BMI, but not ASMI, were independent explanatory factors for plasma BAIBA concentration. In conclusion, plasma BAIBA concentration is inversely correlated with indexes of fat mass, indicating that BAIBA may be a therapeutic target for excessive fat accumulation.




Similar content being viewed by others
Data availability
The data sets generated and/or analyzed during the current study are not publicly available, because a research agreement from all authors is required for data sharing, but are available from the corresponding author on reasonable request.
References
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15(5):288–298
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP, American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council (2021) Obesity and cardiovascular disease: a scientific statement from the american heart association. Circulation 143(21):e984–e1010
Roger VL (2021) Epidemiology of heart failure: a contemporary perspective. Circ Res 128(10):1421–1434
Ebong IA, Goff DC Jr, Rodriguez CJ, Chen H, Bertoni AG (2014) Mechanisms of heart failure in obesity. Obes Res Clin Pract 8(6):e540–e548
Ren J, Wu NN, Wang S, Sowers JR, Zhang Y (2021) Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev 101(4):1745–1807
Reddy YNV, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE, Borlaug BA (2019) Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail 7(8):678–687
Katano S, Yano T, Ohori K, Kouzu H, Nagaoka R, Honma S, Shimomura K, Inoue T, Takamura Y, Ishigo T, Watanabe A, Koyama M, Nagano N, Fujito T, Nishikawa R, Ohwada W, Hashimoto A, Katayose M, Ishiai S, Miura T (2021) Barthel index score predicts mortality in elderly heart failure - a goal of comprehensive cardiac rehabilitation. Circ J 86(1):70–78
Katano S, Yano T, Kouzu H, Ohori K, Shimomura K, Honma S, Nagaoka R, Inoue T, Takamura Y, Ishigo T, Watanabe A, Koyama M, Nagano N, Fujito T, Nishikawa R, Ohwada W, Hashimoto A, Katayose M, Miura T (2021) Energy intake during hospital stay predicts all-cause mortality after discharge independently of nutritional status in elderly heart failure patients. Clin Res Cardiol 110(8):1202–1220
Watanabe A, Katano S, Yano T, Nagaoka R, Numazawa R, Honma S, Yamano K, Fujisawa Y, Ohori K, Kouzu H, Ishigo T, Katayose M, Hashimoto A, Furuhashi M (2022) Loss of perceived social role, an index of social frailty, is an independent predictor of future adverse events in hospitalized patients with heart failure. Front Cardiovasc Med 9:1051570
Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee CH, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP (2022) Exerkines in health, resilience and disease. Nat Rev Endocrinol 18(5):273–289
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE (2014) β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19(1):96–108
Shimba Y, Katayama K, Miyoshi N, Ikeda M, Morita A, Miura S (2020) β-aminoisobutyric acid suppresses atherosclerosis in apolipoprotein E-knockout mice. Biol Pharm Bull 43(6):1016–1019
Sawada M, Yamamoto H, Ogasahara A, Tanaka Y, Kihara S (2019) β-aminoisobutyric acid protects against vascular inflammation through PGC-1β-induced antioxidative properties. Biochem Biophys Res Commun 516(3):963–968
Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM (2015) BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia 58(9):2096–2105
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 37:2129–2200
Ishigo T, Katano S, Yano T, Kouzu H, Ohori K, Nakata H, Nonoyama M, Inoue T, Takamura Y, Nagaoka R, Kondo F, Nakano K, Takada R, Kitagawa M, Kimyo T, Miura T (2020) Overestimation of glomerular filtration rate by creatinine-based equation in heart failure patients is predicted by a novel scoring system. Geriatr Gerontol Int 20(8):752–758
Yamada M, Arai H (2015) Predictive value of frailty scores for healthy life expectancy in community-dwelling older japanese adults. J Am Med Dir Assoc 16(11):1002.e7–11
Kouzu H, Katano S, Yano T, Ohori K, Nagaoka R, Inoue T, Takamura Y, Ishigo T, Watanabe A, Koyama M, Nagano N, Fujito T, Nishikawa R, Ohwada W, Miura T (2021) Plasma amino acid profiling improves predictive accuracy of adverse events in patients with heart failure. ESC Heart Fail 8(6):5045–5056
Katano S, Yano T, Shimizu M, Ohori K, Kouzu H, Koyama M, Nagaoka R, Inoue T, Takamura Y, Ishigo T, Takashima H, Katayose M, Ohnishi H, Miura T (2021) Does renin-angiotensin system inhibition have impacts on muscle mass and bone mineral density in heart failure patients? ESC Heart Fail 8(4):2617–2624
Katano S, Yano T, Tsukada T, Kouzu H, Honma S, Inoue T, Takamura Y, Nagaoka R, Ishigo T, Watanabe A, Ohori K, Koyama M, Nagano N, Fujito T, Nishikawa R, Takashima H, Hashimoto A, Katayose M, Miura T (2020) Clinical risk factors and prognostic impact of osteoporosis in patients with chronic heart failure. Circ J 84(12):2224–2234
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser 843:1–129
Narayan SB, Ditewig-Meyers G, Graham KS, Scott R, Bennett MJ (2011) Measurement of plasma amino acids by ultraperformance® liquid chromatography. Clin Chem Lab Med 49:1177–1185
Peake RW, Law T, Hoover PN, Gaewsky L, Shkreta A, Kellogg MD (2013) Improved separation and analysis of plasma amino acids by modification of the MassTrak™ AAA Solution Ultraperformance® liquid chromatography method. Clin Chim Acta 423:75–82
Stautemas J, Van Kuilenburg ABP, Stroomer L, Vaz F, Blancquaert L, Lefevere FBD, Everaert I, Derave W (2019) Acute aerobic exercise leads to increased plasma levels of R- and S-β-aminoisobutyric acid in humans. Front Physiol 10:1240
Armstrong D, Yates K, Kakimoto Y, Taniguchi K, Kappe T (1963) Excretion of b-aminoisobutyric acid by man. J Biol Chem 238:1447–1455
Short KR, Chadwick JQ, Teague AM, Tullier MA, Wolbert L, Coleman C, Copeland KC (2019) Effect of obesity and exercise training on plasma amino acids and amino metabolites in american indian adolescents. J Clin Endocrinol Metab 104(8):3249–3261
Rietman A, Stanley TL, Clish C, Mootha V, Mensink M, Grinspoon SK, Makimura H (2016) Associations between plasma branched-chain amino acids, β-aminoisobutyric acid and body composition. J Nutr Sci 5:e6
Heymsfield SB, Wadden TA (2017) Mechanisms, pathophysiology, and management of obesity. N Engl J Med 376(3):254–266
Wen X, Zhang B, Wu B, Xiao H, Li Z, Li R, Xu X, Li T (2022) Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct Target Ther 7(1):298
Cheng L, Wang J, Dai H, Duan Y, An Y, Shi L, Lv Y, Li H, Wang C, Ma Q, Li Y, Li P, Du H, Zhao B (2021) Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 10(1):48–65
Whitehead A, Krause FN, Moran A, MacCannell ADV, Scragg JL, McNally BD, Boateng E, Murfitt SA, Virtue S, Wright J, Garnham J, Davies GR, Dodgson J, Schneider JE, Murray AJ, Church C, Vidal-Puig A, Witte KK, Griffin JL, Roberts LD (2021) Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat Commun 12(1):1905
Serra D, Mera P, Malandrino MI, Mir JF, Herrero L (2013) Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal 19(3):269–284
Krieger JM, Hagele AM, Orr LS, Walden KE, Gross KN, Mumford PW, Kerksick CM (2022) Dose-response absorption kinetics of oral L-beta-aminoisobutyric acid (L-BAIBA) supplementation in healthy men and women. J Diet Suppl. https://doi.org/10.1080/19390211.2022.2128141
Morales FE, Forsse JS, Andre TL, McKinley-Barnard SK, Hwang PS, Anthony IG, Tinsley GM, Spillane M, Grandjean PW, Ramirez A, Willoughby DS (2017) BAIBA does not regulate UCP-3 expression in human skeletal muscle as a response to aerobic exercise. J Am Coll Nutr 36(3):200–209
Li VL, He Y, Contrepois K, Liu H, Kim JT, Wiggenhorn AL, Tanzo JT, Tung AS, Lyu X, Zushin PH, Jansen RS, Michael B, Loh KY, Yang AC, Carl CS, Voldstedlund CT, Wei W, Terrell SM, Moeller BC, Arthur RM, Wallis GA, van de Wetering K, Stahl A, Kiens B, Richter EA, Banik SM, Snyder MP, Xu Y, Long JZ (2022) An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606(7915):785–790
Packer M (2022) Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 146(18):1383–1405
Otsuka H, Yokomizo H, Nakamura S, Izumi Y, Takahashi M, Obara S, Nakao M, Ikeda Y, Sato N, Sakamoto R, Miyachi Y, Miyazawa T, Bamba T, Ogawa Y (2022) Differential effect of canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on slow and fast skeletal muscles from nondiabetic mice. Biochem J 479(3):425–444
Nambu H, Takada S, Fukushima A, Matsumoto J, Kakutani N, Maekawa S, Shirakawa R, Nakano I, Furihata T, Katayama T, Yamanashi K, Obata Y, Saito A, Yokota T, Kinugawa S (2022) Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. Eur J Pharmacol 866:172810
Katano S, Yano T, Kouzu H, Nagaoka R, Numazawa R, Yamano K, Fujisawa Y, Ohori K, Nagano N, Fujito T, Nishikawa R, Ohwada W, Katayose M, Sato T, Kuno A, Furuhashi M (2022) Elevated circulating level of β-aminoisobutyric acid (BAIBA) in heart failure patients with type 2 diabetes receiving sodium-glucose cotransporter 2 inhibitors. Cardiovasc Diabetol 21(1):285
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813(7):1269–1278
Carbone S, Lavie CJ, Arena R (2017) Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc 92:266–279
Padwal R, McAlister FA, McMurray JJ, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C, Earle N, Whalley G, Poppe KK, Doughty RN, Bayes-Genis A, Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) (2014) The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond) 38(8):1110–1114
Kapoor JR, Heidenreich PA (2010) Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J 159(1):75–80
von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T, Haarmann H, Schefold JC, Lainscak M, Cleland JGF, Doehner W, Hasenfuss G, Anker SD (2020) Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle 11(5):1242–1249
Konishi M, Akiyama E, Matsuzawa Y, Sato R, Kikuchi S, Nakahashi H, Maejima N, Iwahashi N, Kosuge M, Ebina T, Hibi K, Misumi T, von Haehling S, Anker SD, Tamura K, Kimura K (2021) Prognostic impact of muscle and fat mass in patients with heart failure. J Cachexia Sarcopenia Muscle 12(3):568–576
Katano S, Honma S, Nagaoka R, Numazawa R, Yamano K, Fujisawa Y, Ohori K, Kouzu H, Hashimoto A, Katayose M, Yano T (2022) Anthropometric parameters-derived estimation of muscle mass predicts all-cause mortality in heart failure patients. ESC Heart Fail 9(6):4358–4365
Ohori K, Yano T, Katano S, Kouzu H, Honma S, Shimomura K, Inoue T, Takamura Y, Nagaoka R, Koyama M, Nagano N, Fujito T, Nishikawa R, Ishigo T, Watanabe A, Hashimoto A, Miura T (2021) High percent body fat mass predicts lower risk of cardiac events in patients with heart failure: an explanation of the obesity paradox. BMC Geriatr 21(1):16
Tanianskii DA, Jarzebska N, Birkenfeld AL, O’Sullivan JF, Rodionov RN (2019) Beta-aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients 11(3):524
Furuhashi M (2019) Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb 26(3):216–232
Rallidis LS, Katsimardos A, Kosmas N, Rallidi T, Zapantiotis D, Varounis C, Kountouri A (2022) Differential prognostic value of resistin for cardiac death in patients with coronary artery disease according to the presence of metabolic syndrome. Heart Vessels 37(5):713–719
Cretton EM, Zhou Z, Kidd LB, McClure HM, Kaul S, Hitchcock MJ, Sommadossi JP (1993) In vitro and in vivo disposition and metabolism of 3’-deoxy-2’,3’-didehydrothymidine. Antimicrob Agents Chemother 37(9):1816–1825
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katano, S., Yano, T., Kouzu, H. et al. Circulating level of β-aminoisobutyric acid (BAIBA), a novel myokine-like molecule, is inversely associated with fat mass in patients with heart failure. Heart Vessels 39, 35–47 (2024). https://doi.org/10.1007/s00380-023-02308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-023-02308-y