Abstract
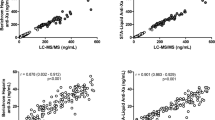
Direct oral anticoagulants, including edoxaban, primarily do not need routine monitoring of the anticoagulant effect. However, extremely high/low plasma concentrations of edoxaban (PC-Ed) should be properly evaluated, especially when patients under anticoagulation therapy are at an emergency state. For this purpose, PC-Ed determined by an anti-Xa assay (indirect PC-Ed) is more convenient and, therefore, more useful compared with PC-Ed determined by an LC–MS/MS (direct PC-Ed) in daily clinical practice. Consecutive 97 patients with non-valvular atrial fibrillation (NVAF) under edoxaban therapy were evaluated, in whom edoxaban 60/30 mg doses were prescribed for 48/49 patients, 71 (73.2%) were men, and the average age was 69 years. CHADS2 score 0, 1, and ≥ 2 were 26.8%, 44.3%, and 28.9%, while CHA2DS2-VASc score 0, 1, and ≥ 2 were 14.4%, 16.5%, and 69.1%, respectively. Median values of direct and indirect PC-Ed by LC–MS/MS and anti-Xa assay were 187.1 and 176.1 ng/mL at peak (2–4 h post-dose) and 14.4 and 17.5 ng/mL at trough (pre-dose), respectively. The PC-Ed at peak and trough by two methods were significantly correlated, and the correlation coefficients were r = 0.973 and 0.963 (both, p < 0.0001), respectively. By a Bland–Altman plot, mean differences between the direct and indirect PC-Ed [lower to upper percent limit of agreement] were − 4.87 [− 46.71 to 36.98] and 4.66 [− 1.37 to 10.69] ng/mL at peak and trough, respectively. Moreover, mean % error for difference between the direct and indirect PC-Ed [lower to upper percent limit of agreement] was − 1.22 [− 20.59 to 18.14] and 31.75 [− 14.03 to 77.53] % at peak and trough, respectively, where the % error extremely increased around the lower limit of detection (LLOD) in the anti-Xa assay. Strong similarity was observed between the direct and indirect PC-Ed, especially at peak. The indirect PC-Ed was higher than the direct PC-Ed, especially around the LLOD, suggesting the need for caution when we use the anti-Xa assay for measurement of trough PC-Ed (UMIN 000032492).




Similar content being viewed by others
Change history
28 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00380-023-02338-6
References
Furugohri T, Isobe K, Honda Y, Kamisato-Matsumoto C, Sugiyama N, Nagahara T, Morishima Y, Shibano T (2008) DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 6:1542–1549
Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, Deenadayalu N, Jarolim P, Betcher J, Shi M, Brown K, Patel I, Mercuri M, Antman EM (2015) Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385:2288–2295
Cuker A, Husseinzadeh H (2015) Laboratory measurement of the anticoagulant activity of edoxaban: a systematic review. J Thromb Thrombolysis 39:288–294
Douxfils J, Chatelain B, Chatelain C, Dogne JM, Mullier F (2016) Edoxaban: Impact on routine and specific coagulation assays. A practical laboratory guide. Thromb Haemost 115:368–381
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, ESC Scientific Document Group (2018) The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39:1330–1393
Cuker A (2016) Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis 41:241–247
Kodani E, Okumura K, Inoue H, Yamashita T, Atarashi H, Origasa H (2011) Present status and problems of international sensitivity index (ISI) on the measurement of international normalized ratio of prothrombin time (INR). Jpn J Electrocardiol 31:225–233
Suzuki S, Morishima Y, Takita A, Yagi N, Otsuka T, Arita T, Yamashita T (2019) Responses of prothrombin time and activated partial thromboplastin time to edoxaban in Japanese patients with non-valvular atrial fibrillation: characteristics of representative reagents in Japan (CVI ARO 7). Heart Vessels. https://doi.org/10.1007/s00380-019-01438-6
J-ELD AF study investigators (2019) Investigation on efficacy and safety of Apixaban in Japanese elderly patients: Investigator-Initiated Multicenter Prospective Cohort Study (J-ELD AF [CVI ARO 5] Study) [UMIN000017895] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000020733. Accessed 15 May 2019.
Akao M, Yamashita T, Okumura K, J-ELDAF Investigators (2016) Study design of J-ELD AF: a multicenter prospective cohort study to investigate the efficacy and safety of apixaban in Japanese elderly patients. J Cardiol 68:554–558
CVI ARO 1 investigators (2019) An analysis on distribution and inter-relationships of biomarkers under rivaroxaban in Japanese patients with non-valvular atrial fibrillation (CVI ARO 1 study) [UMIN000016424] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000019074. Accessed 15 May 2019.
Suzuki S, Yamashita T, Kasai H, Otsuka T, Sagara K (2017) Response of prothrombin time to rivaroxaban in Japanese patients with non-valvular atrial fibrillation: characteristics of 5 representative reagents in Japan (CVI ARO 1). Thromb Res 150:73–75
Suzuki S, Yamashita T, Kasai H, Otsuka T, Sagara K (2018) An analysis on distribution and inter-relationships of biomarkers under rivaroxaban in Japanese patients with non-valvular atrial fibrillation (CVI ARO 1). Drug Metab Pharmacokinet 33:188–193
R-mark study investigators (2019) An analysis on validity of distribution of biomarkers, exploration of correlation between biomarker outliers and adverse events under rivaroxaban in Japanese patients with non-valvular atrial fibrillation (R-mark [CVI ARO 2] study) [UMIN000022721] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000024525. Accessed 15 May 2019.
CVI ARO 9 investigators (2019) The effect of landiolol on rehospitalization for heart failure in patients with acute heart failure and atrial fibrillation (CVI ARO 9 study) [UMIN000020290] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000020641. Accessed 15 May 2019.
CVI ARO 3 investigators (2019) An analysis on validity of monitoring by wristwatch-type sphygmograph in detecting of atrial fibrillation (CVI ARO 3a study) [UMIN000022722] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000025921. Accessed 15 May 2019.
CVI ARO 3 investigators (2019) An analysis on usefulness of monitoring by wristwatch-type sphygmograph in evaluation of recurrence of atrial fibrillation after catheter ablation (CVI ARO 3b study) [UMIN000022723] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000025937. Accessed 15 May 2019.
CVI ARO 3 investigators (2019) A pilot study of detection of asymptomatic atrial fibrillation using wristwatch-type sphygmograph among patients with sinus rhythm and high CHADS2 score (CVI ARO 3c study) [UMIN000022724] [web page]. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000025940.Accessed 15 May 2019.
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J (2012) Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos 40:2250–2255
He L, Kochan J, Lin M, Vandell A, Brown K, Depasse F (2017) Determination of edoxaban equivalent concentrations in human plasma by an automated anti-factor Xa chromogenic assay. Thromb Res 155:121–127
Altman DG, Bland JM (1983) Measurement in Medicine: The Analysis of Method Comparison Studies. Statistician 32:307–317
Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogne JM, Mullier F (2013) Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 110:723–731
Konigsbrugge O, Quehenberger P, Belik S, Weigel G, Seger C, Griesmacher A, Pabinger I, Ay C (2015) Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol 94:1463–1471
Studt JD, Alberio L, Angelillo-Scherrer A, Asmis LM, Fontana P, Korte W, Mendez A, Schmid P, Stricker H, Tsakiris DA, Wuillemin WA, Nagler M (2017) Accuracy and consistency of anti-Xa activity measurement for determination of rivaroxaban plasma levels. J Thromb Haemost 15:1576–1583
Gosselin RC, Adcock Funk DM, Taylor JM, Francart SJ, Hawes EM, Friedman KD, Moll S (2014) Comparison of anti-Xa and dilute Russell viper venom time assays in quantifying drug levels in patients on therapeutic doses of rivaroxaban. Arch Pathol Lab Med 138:1680–1684
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was financially supported by Daiichi Sankyo. Dr. Suzuki received research funding from Daiichi Sankyo and Mitsubishi-Tanabe. Dr. Yamashita received research funding from Boehringer Ingelheim and Daiichi Sankyo, and remuneration from Boehringer Ingelheim, Daiichi Sankyo, Bayer Healthcare, Pfizer, Bristol-Myers Squibb, Eisai and Ono Pharmaceutical. Dr. Morishima and Mr. Takita are employees of Daiichi Sankyo.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suzuki, S., Morishima, Y., Takita, A. et al. Association between plasma concentration of edoxaban determined by direct and indirect methods in Japanese patients with non-valvular atrial fibrillation (CVI ARO 7). Heart Vessels 35, 409–416 (2020). https://doi.org/10.1007/s00380-019-01501-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01501-2