Abstract
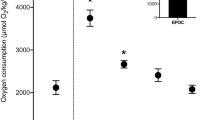
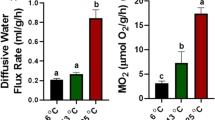
In the traditional osmorespiratory compromise, fish increase their effective gill permeability to O2 during exercise or hypoxia, and in consequence suffer unfavorable ionic and osmotic fluxes. However oscars, which live in the frequently hypoxic ion-poor waters of the Amazon, actually decrease ionic fluxes across the gills during acute hypoxia without changing gill paracellular permeability, and exhibit rapid paving over of the mitochondrial-rich cells (MRCs). But what happens during prolonged exercise? Gill paracellular permeability, ionic fluxes, and gill morphology were examined in juvenile oscars at rest and during aerobic swimming. Initial validation tests with urinary catheterized fish quantified drinking, glomerular filtration, and urinary flow rates, and confirmed that measurements of gill paracellular permeability as [3H]PEG-4000 clearances were the same in efflux and influx directions, but far lower than previously measured in comparably sized trout. Although the oscars achieved a very similar proportional increase (90 %) in oxygen consumption (MO2) to trout during steady-state swimming at 1.2 body lengths sec−1, there was no increase in gill paracellular permeability, in contrast to trout. However, oscars did exhibit increased unidirectional Na+ efflux and net K+ rates during exercise, but no change in drinking rate. There were no changes in MRC numbers or exposure, or other alterations in gill morphology during exercise. A substantial interlamellar cell mass (ILCM) that covered the lamellae to a depth of 30 % was unchanged by 4 h of swimming activity. We conclude that a low branchial paracellular permeability which can be dissociated from changes in O2 flux, as well as the presence of the ILCM, may be adaptive in limiting ionoregulatory costs for a species endemic to ion-poor, frequently hypoxic waters.





Similar content being viewed by others
References
Almeida-Val VMF, Hochachka PW (1995) Air-breathing fishes: metabolic biochemistry of the first diving vertebrates. In: Mommsen TP (ed) Hochachka PW. Environmental and ecological biochemistry, Amsterdam, pp 45–55
Almeida-Val VMF, Paula-Silva MN, Duncan WP, Lopes NP, Val AL, Land SC (1999) Increase of anaerobic potential during growth of an Amazonian cichlid, Astronotus ocellatus. Survivorship and LDH regulation after hypoxia exposure. In: Val AL, Almeida-Val VMF (eds) Biology of tropical fishes. INPA, Manaus, pp 437–448
Almeida-Val VMF, Val AL, Duncan WP, Souza FCA, Paula-Silva MN, Land SC (2000) Scaling effects on hypoxia tolerance in the Amazon fish Astronotus ocellatus (Perciformes: Cichlidae): contribution of tissue enzyme levels. Comp Biochem Physiol B 125:219–226
Barnes KR, Cozzi RR, Robertson G, Marshall WS (2014) Cold acclimation of NaCl secretion in a eurythermic teleost: mitochondrial function and gill remodeling. Comp Biochem Physiol A 168:50–62
Boutilier RG, Heming TA, Iwama GK (1984) Appendix: physicochemical parameters for use in fish respiratory physiology. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 10. Academic Press, New York, pp 403–430
Brauner CJ, Matey V, Zhang W, Richards JG, Dhillon R, Cao ZD, Fu S-J (2011) Gill remodeling in crucian carp during sustained exercise and the effect on subsequent swimming performance. Physiol Biochem Zool 84:535–542
Chapman LJ, Galis F, Shinn J (2000) Phenotypic plasticity and the possible role of genetic assimilation: hypoxia-induced trade-offs in the morphological traits of an African cichlid. Ecol Lett 3:387–393
Chippari-Gomes AR, Gomes LC, Lopes NP, Val AL, Almeida-Val VM (2005) Metabolic adjustments in two Amazonian cichlids exposed to hypoxia and anoxia. Comp Biochem Physiol B 141:347–355
Clarke AJ, Johnson NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Conte FP (1969) Salt secretion. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 1. Academic Press, New York, pp 241–292
De Boeck G, Wood CM, Iftikar FI, Matey V, Scott GR, Sloman KA, da Silva MDNP, Almeida-Val VMF, Val AL (2013) Interactions between hypoxia tolerance and food deprivation in Amazonian oscars, Astronotus ocellatus. J Exp Biol 216:4590–4600
Dhillon RS, Yao L, Matey V, Chen B-J, Zhang AJ, Cao Z-D, Fu S-J, Brauner CJ, Wang YS, Richards JG (2013) Interspecific differences in hypoxia-induced gill remodeling in carp. Physiol Biochem Zool 86:727–739
Gonzalez RJ, McDonald DG (1992) The relationship between oxygen consumption and ion loss in a freshwater fish. J Exp Biol 163:317–332
Gonzalez RJ, McDonald DG (1994) The relationship between oxygen uptake and ion loss in fish from diverse habitats. J Exp Biol 190:95–108
Hickman C, Trump B (1969) Kidney. In: Hoar WS, Randall D (eds) Fish physiology, vol 1. Academic Press, New York, pp 211–212
Hofmann EL, Butler DG (1979) The effect of increased metabolic rate on renal function in the rainbow trout, Salmo gairdneri. J Exp Biol 82:11–23
Iftikar F, Matey V, Wood CM (2010) The ionoregulatory responses to hypoxia in the freshwater rainbow trout Oncorhynchus mykiss. Physiol Biochem Zool 83:343–355
Johannsson OE, Bergman HL, Wood CM, Laurent P, Kavembe DG, Bianchini A, Maina JN, Chevalier C, Bianchini LF, Papah MB, Ojoo RO (2014) Air breathing in the Lake Magadi tilapia Alcolapia grahami, under normoxic and hyperoxic conditions, and the association with sunlight and ROS. J Fish Biol 84:844–863
Karnovsky MJ (1965) A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–139
Matey V, Richards JG, Wang Y, Wood CM, Rogers J, Davies R, Murray BW, Chen XQ, Du J, Brauner CJ (2008) The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J Exp Biol 211:1063–1074
Matey V, Iftikar FI, De Boeck G, Scott GR, Sloman KA, Almeida-Val VMF, Val AL, Wood CM (2011) Gill morphology and acute hypoxia: responses of mitochondria-rich, pavement, and mucous cells in two species with very different approaches to the osmo-respiratory compromise, the Amazonian oscar (Astronotus ocellatus) and the rainbow trout. Can J Zool 89:307–324
Muusze B, Marcon J, van den Thillart G, Almeida-Val VMF (1998) Hypoxia tolerance of Amazon fish. Respirometry and energy metabolism of the cichlid Astronotus ocellatus. Comp Biochem Physiol A 120:151–156
Nilsson S (1986) Control of gill blood flow. In: Nilsson S, Holmgren S (eds) Fish physiology: recent advances. Croom Helm, London, pp 87–101
Nilsson GE (2007) Gill remodelling in fish—a new fashion or an ancient secret? J Exp Biol 210:2403–2409
Ong KJ, Stevens ED, Wright PA (2007) Gill morphology of the mangrove killifish (Kryptolebias marmoratus) is plastic and changes in response to terrestrial air exposure. J Exp Biol 210:1109–1115
Perry SF, Schwaiger T, Kumai Y, Tzaneva V, Braun MH (2010) The consequences of reversible gill remodeling on ammonia excretion in goldfish (Carassius auratus). J Exp Biol 213:3656–3665
Perry SF, Fletcher C, Bailey S, Ting J, Bradshaw J, Tzaneva V, Gilmour KM (2012) The interactive effects of exercise and gill remodeling in goldfish (Carassius auratus). J Comp Physiol B 182:935–945
Postlethwaite E, McDonald DG (1995) Mechanisms of Na and Cl regulation in freshwater-adapted rainbow trout (Oncorynchus mykiss) during exercise and stress. J Exp Biol 198:295–304
Randall DJ, Baumgarten D, Malyusz M (1972) The relationship between gas and ion transfer across the gills of fishes. Comp Biochem Physiol A 41:629–637
Richards JG, Wang YS, Brauner CJ, Gonzalez RJ, Patrick ML, Schulte PM, Choppari-Gomes AR, Almeida-Val VMF, Val AL (2007) Metabolic and ionoregulatory responses of the Amazonian cichlid, Astronotus ocellatus, to severe hypoxia. J Comp Physiol B 177:361–374
Robertson LM, Wood CM (2014) Measuring gill paracellular permeability with polyethelene glycol-4000 in freely swimming trout: proof of principle. J Exp Biol 217:1425–1429
Robertson LM, Val AL, Almeida-Val VF, Wood CM (2015) Ionoregulatory aspects of the osmorespiratory compromise during acute environmental hypoxia in 12 tropical and temperate teleosts. Physiol Biochem Zool 88:357–370
Scott GR, Wood CM, Sloman KA, Iftikar FI, De Boeck G, Almeida-Val VMF, Val AL (2008) Respiratory responses to progressive hypoxia in the Amazonian oscar, Astronotus ocellatus. Respir Physiol Neurobiol 162:109–116
Sloman KA, Wood CM, Scott GR, Wood S, Kajimura M, Johannsson OE, Almeida-Val VMF, Val AL (2006) Tribute to R. G. Boutilier: the effect of size on the physiological and behavioural responses of oscar, Astronotus ocellatus, to hypoxia. J Exp Biol 209:1197–1205
Smith AA, Zimmer AM, Wood CM (2012) Branchial and extra branchial ammonia excretion in goldfish (Carassius auratus) following thermally induced gill remodelling. Comp Biochem Physiol A 162:185–192
Sollid J, Nilsson GE (2006) Plasticity of respiratory structures—adaptive remodelling of fish gills induced by ambient oxygen and temperature. Respir Physiol Neurobiol 154:241–251
Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206:3667–3673
Sollid J, Weber RE, Nilsson GE (2005) Temperature alters the respiratory surface area of Crucian carp Carassiuscarassius and goldfish Carassiusauratus. J Exp Biol 208:1109–1116
Stevens ED (1972) Changes in body weight caused by handling and exercise in fish. J Fish Res Bd Can 29:202–203
Thomas S, Fievet B, Motais R (1986) Effect of deep hypoxia on acid-base balance in trout: role of ion transfer processes. Am J Physiol 250:R319–R327
Val AL, Almeida-Val VMF (1995) Fishes of the Amazon and their environment. Physiological and biochemical features. Springer, Heidelberg, p 224
Verdouw H, van Echted CJA, Dekkers EMJ (1978) Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12:399–402
Wilson RW, Gilmour KM, Henry RP, Wood CM (1996) Intestinal base excretion in the seawater-adapted rainbow trout: a role in acid-base balance? J Exp Biol 199:2331–2343
Wolf K (1963) Physiological salines for fresh-water teleosts. Prog Fish Cult 25:135–140
Wood CM (1988) Acid-base and ionic exchanges at gills and kidney after exhaustive exercise in the rainbow trout. J Exp Biol 146:461–481
Wood CM (1992) Flux measurements as indices of H+ and metal effects on freshwater fish. Aquat Toxicol 22:239–264
Wood CM, Patrick ML (1994) Methods for assessing kidney and urinary bladder function in fish. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 3. Elsevier, New York, pp 127–143
Wood CM, Randall DJ (1973a) The influence of swimming activity on sodium balance in the rainbow trout (Salmo gairdneri). J Comp Physiol 82:207–233
Wood CM, Randall DJ (1973b) Sodium balance in the rainbow trout (Salmo gairdneri) during extended exercise. J Comp Physiol 82:235–256
Wood CM, Randall DJ (1973c) The influence of swimming activity on water balance in the rainbow trout (Salmo gairdneri). J Comp Physiol 82:257–276
Wood CM, Kajimura M, Sloman KA, Scott GR, Walsh PJ, Almeida-Val VMF, Val AL (2007) Rapid regulation of Na+ fluxes and ammonia excretion in response to acute environmental hypoxia in the Amazonian oscar, Astronotus ocellatus. Am J Physiol 292:R2048–R2058
Wood CM, Iftikar FI, Scott GR, De Boeck G, Sloman KA, Matey V, Valdez Domingos FX, Duarte RM, Almeida-Val VMF, Val AL (2009) Regulation of gill transcellular permeability and renal function during acute hypoxia in the Amazonian oscar (Astronotus ocellatus): new angles to the osmo respiratory compromise. J Exp Biol 212:949–964
Acknowledgments
Supported in Manaus, Brazil by FAPEAM and CNPq through the INCT-ADAPTA grant to ALV, and Ciência sem Fronteiras grant to ALV and CMW, in Rio Grande, Brazil by an award from the International Development Research Centre (IDRC) and the Canada Research Chair Program to AB and CMW, and an award from CNPq to AB in the scope of INCT-TA, and in Canada by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant to CMW. LMR was supported by an Ontario Graduate Scholarship. DK was a recipient of a PhD fellowship from CNPq. CMW was supported by the Canada Research Chairs program, and is the recipient of a fellowship from the Science Without Borders Program (CNPq-Brazil). ALV, VFAV, and AB are recipients of research fellowships from CNPq, and AB is also supported by the International Research Chair Program of IDRC. We thank two anonymous reviewers for constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Robertson, L.M., Kochhann, D., Bianchini, A. et al. Gill paracellular permeability and the osmorespiratory compromise during exercise in the hypoxia-tolerant Amazonian oscar (Astronotus ocellatus). J Comp Physiol B 185, 741–754 (2015). https://doi.org/10.1007/s00360-015-0918-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0918-4