Abstract
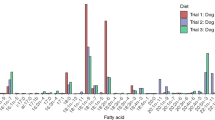
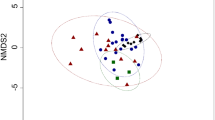
Phocids routinely fast for extended periods. During these fasts, energetic requirements are met primarily through the catabolism of blubber lipid. To assess whether fatty acid (FA) composition changes during the postweaning fast in northern elephant seals, blubber biopsies were acquired longitudinally from 43 pups at 2.3 ± 1.5 and 55.2 ± 3.7 days postweaning in 1999 and 2000. At weaning, short-chain monounsaturated FA (SC-MUFA, ≤18 carbons) dominated the blubber while saturated FA (SFA) were found in the next highest proportion. The major FA (all ≥1 % by mass) comprised approximately 91 % of total blubber FA. In both years, 18:1n-9 and 16:0 were the most prevalent FA. Major FA mobilized during the fast consisted of polyunsaturated FA (PUFA), SFA, and SC-MUFA. Long-chain MUFA (>18 carbons) tended to be conserved. The fractional mobilization value of 20:5n-3 was the highest, resulting in significant reductions of this PUFA. Although concentrations of some blubber FA changed significantly during the postweaning fast, the general FA signature of blubber was similar at weaning and near the end of the fast. Changes in some FA differed across years. For example, the concentration of 20:4n-6, a minor PUFA, was significantly reduced in 1999 but not in 2000. FA mobilization patterns in northern elephant seal pups are somewhat similar to those reported previously for other fasting phocids and terrestrial mammals, though there are some notable differences. Differences in FA mobilization patterns across mammalian species may be related to differences in diets, geographical distribution, environmental factors, physiological adaptations, and life history stage.



Similar content being viewed by others
References
Ackman RG, Eaton CA, Jangaard PM (1965) Lipids of the fin whale (Balaenoptera physalus) from north Atlantic waters. I. Fatty acid composition of whole blubber and blubber sections. Can J Biochem 43:1513–1520
Ackman RG, Hingley JH, Eaton CA, Sipos JC, Mitchell ED (1975a) Blubber fat deposition in mysticeti whales. Can J Zool 53:1332–1339
Ackman RG, Hingley JH, Eaton CA, Logan VH, Odense PH (1975b) Layering and tissue composition in the blubber of the northwest Atlantic sei whale (Balaenoptera borealis). Can J Zool 53:1340–1344
Aguilar A, Borrell A (1990) Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J Mammal 71:544–554
American Society of Mammalogists—Society’s Committee on Marine Mammals, Scheffer VB (ed) (1967) Standard measurements of seals. J Mammal 48: 459–462
Arnbom T, Fedak MA, Boyd IL, McConnell BJ (1993) Variation in weaning mass of pups in relation to maternal mass, postweaning fast duration, and weaned pup behaviour in southern elephant seals (Mirounga leonina) at South Georgia. Can J Zool 71:1772–1781
Atkinson G (2001) Analysis of repeated measurements in physical therapy research. Phys Ther Sport 2:194–208
Best NJ, Bradshaw CJA, Hindell MA, Nichols PD (2003) Vertical stratification of fatty acids in the blubber of southern elephant seals (Mirounga leonina): implications for diet analysis. Comp Biochem Physiol B 134:253–263
Bradshaw CJA, Hindell MA, Best NJ, Phillips KL, Wilson G, Nichols PD (2003) You are what you eat: describing the foraging ecology of southern elephant seals (Mirounga leonina): using blubber fatty acids. Proc R Soc Lond B 270:1283–1292
Bryden MM, Stokes GB (1969) Metabolism of fatty acids in the southern elephant seal Mirounga leonina (L.). Can J Biochem 47:757–760
Castellini MA, Costa DP, Huntley AC (1987) Fatty acid metabolism in fasting elephant seal pups. J Comp Physiol B 157:445–449
Castellini MA, Trumble SJ, Mau TL, Yochem PK, Stewart BS, Koski MA (2009) Body and blubber relationships in Antarctic pack ice seals: implications for blubber depth patterns. Physiol Biochem Zool 82:113–120
Conner WE, Lin DS, Colvis C (1996) Differential mobilization of fatty acids from adipose tissue. J Lipid Res 37:290–298
Costa DP (1993) The relationship between reproductive and foraging energetics and the evolution of the Pinnipedia. In: Boyd IL (ed) Marine mammals: advances in behavioural and population biology, vol 66. Symposium Zoological Society of London, Oxford University Press, New York, pp 293–314
Crocker DE, Williams JD, Costa DP, Le Boeuf BJ (2001) Maternal traits and reproductive effort in northern elephant seals. Ecol 82:3541–3555
Crocker DE, Costa DP, Le Boeuf BJ, Webb PM, Houser DS (2006) Impact of El Niño on the foraging behavior of female northern elephant seals. Mar Ecol Prog Ser 309:1–10
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Rev 20:649–688
Deutsch CJ, Crocker DE, Costa DP, Le Boeuf BJ (1994) Sex- and age-related variation in reproductive effort of northern elephant seals. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behavior and physiology. University of California Press, Berkeley, pp 169–210
Filzmoser P, Hron K, Reimann C (2009) Univariate statistical analysis of environmental (compositional) data: problems and possibilities. Sci Total Environ 407:6100–6108
Folch J, Lees M, Sloan-Stnaley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Groscolas R, Robin J-P (2001) Long-term fasting and re-feeding in penguins. Comp Biochem Physiol A 128:645–655
Guglielmo CG (2010) Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr Comp Biol 50:336–345
Irving L, Schmidt-Nielsen K, Abrahamsen NSB (1957) On the melting points of animal fats in cold climates. Physiol Zool 30:93–105
Iverson SJ (2002) Blubber. In: Perrin WF, Wursig B, Thewissen HGM (eds) Encyclopedia of marine mammals. Academic Press, San Diego, pp 107–112
Iverson SJ, Sampugna J, Oftedal OT (1992) Positional specificity of gastric hydrolysis of long- chain n-3 polyunsaturated fatty acids of seal milk triglycerides. Lipids 27:870–878
Iverson SJ, Oftedal OT, Bowen WD, Boness DJ, Sampugna J (1995) Prenatal and postnatal transfer of fatty acids from mother to pup in the hooded seal. J Comp Physiol B 156:1–12
Iverson SJ, Arnould JPY, Boyd IL (1997a) Milk fatty acid signatures indicate both major and minor shifts in the diet of lactating Antarctic fur seals. Can J Zool 75:188–197
Iverson SJ, Frost KJ, Lowry LF (1997b) Fatty acid signatures reveal fine scale structure of foraging distribution of harbor seals and their prey in Prince William Sound, Alaska. Mar Ecol Prog Ser 151:255–271
Iverson SJ, Lang SLC, Cooper M (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36:1283–1287
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Käkelä R, Hyvärinen H (1996a) Fatty acids in extremity tissues of Finnish beavers (Castor canadensis and Castor fiber) and muskrats (Ondatra zibethicus). Comp Biochem Physiol B 113:113–124
Käkelä R, Hyvärinen H (1996b) Site-specific fatty acid composition in adipose tissues of several northern aquatic and terrestrial mammals. Comp Biochem Physiol B 115:501–514
Kirsch PE, Iverson SJ, Bowen WD (2000) Effect of a low-fat diet on body composition and blubber fatty acids of captive juvenile harp seals (Phoca groenlandica). Physiol Biochem Zool 73:45–59
Lockyer C (1991) Body composition of the sperm whale, Physeter catodon, with special reference to the possible functions of depot fats. Rit Fisk 12:1–24
Lockyer CH, McConnell LC, Waters TD (1984) The biochemical composition of fin whale blubber. Can J Zool 62:2553–2562
Mustonen A-M, Käkelä R, Käkelä A, Pyykönen T, Aho J, Nieminen P (2007a) Lipid metabolism in the adipose tissue of a carnivore, the raccoon dog, during prolonged fasting. Exp Biol Med 232:58–69
Mustonen A-M, Asikainen J, Aho J, Nieminen P (2007b) Selective seasonal fatty acid accumulation and mobilization in the wild raccoon dog (Nyctereutes procyonoides). Lipids 42:1155–1167
Nieminen P, Käkelä R, Pyykönen T, Mustonen A-M (2006) Selective fatty acid mobilization in the American mink (Mustela vison) during food deprivation. Comp Biochem Physiol B 145:81–93
Noren DP (2002) Body energy reserve utilization during the postweaning fast of northern elephant seals (Mirounga angustirostris): implications for survival dissertation. University of California, Santa Cruz
Noren DP, Crocker DE, Williams TM, Costa DP (2003) Energy reserve utilization of northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. J Comp Physiol B 173:443–454
Ortiz CL, Costa D, Le Boeuf BJ (1978) Water and energy flux in elephant seal pups fasting under natural conditions. Physiol Zool 51:166–178
Pernia SD (1984) Protein turnover and nitrogen metabolism during long term fasting in northern elephant seal pups dissertation. University of California, Santa Cruz
Pettinger JD (2000) Fatty acid signatures as indicators of foraging ecology of northern elephant seals (Mirounga angustirostris). MSc Thesis, University of California, Santa Cruz
Phinney SD, Tang AB, Johnson SB, Holman RT (1990) Reduced adipose tissue 18:3n-3 with weight loss by very low calorie dieting. Lipids 25:798–806
Pond CM (1978) Morphological aspects and the ecological and mechanical consequences of fat deposition in wild vertebrates. Annu Rev Ecol Syst 9:519–570
Price ER, Krokfors A, Guglielmo CG (2008) Selective mobilization of fatty acids from adipose tissue in migratory birds. J Exp Biol 211:29–34
Raclot T (2003) Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog Lipid Res 42:257–288
Raclot T, Groscolas R (1995) Selective mobilization of adipose tissue fatty acids during energy depletion in the rat. J Lipid Res 36:2164–2173
Rea L, Groscolas R, Mioskowski E, Castellini M (1997) Changes in plasma fatty acids indicate change in nutritional status in developing Weddell seal pups. Polar Biol 18:351–357
Reilly JJ (1991) Adaptations to prolonged fasting in free-living weaned gray seal pups. Am J Physiol 260:R267–R272
Ryg M, Smith TG, Øritsland NA (1988) Thermal significance of the topographical distribution of blubber in ringed seals (Phoca hispidia). Can J Fish Aquat Sci 45:985–992
Smith WL, Murphy RC (2002) The eicosanoids: cyclooxygenase, lipoxygenase and epoxygenase pathways. In: Biochemistry of lipids, lipoproteins and membranes. Elsevier, Oxford, pp 341–371
Sokolov W (1962) Adaptations of the mammalian skin to the aquatic mode of life. Nature 195:464–466
Strandberg U, Käkelä A, Lydersen C, Kovacs KM, Grahl-Nielsen O, Hyvärinen H, Käkelä R (2008) Stratification, composition, and function of marine mammal blubber: the ecology of fatty acids in marine mammals. Physiol Biochem Zool 81:473–485
Strandberg U, Sipilä T, Koskela J, Kunnasranta M, Käkelä R (2011) Vertical fatty acid profiles in blubber of a freshwater ringed seal—Comparison to a marine relative. J Exp Mar Biol Ecol 407:256–265
Trumble SJ, Kanatous SB (2012) Fatty acid use in diving mammals: more than merely fuel. Front Physiol 3:184. doi:10.3389/fphys.2012.00184
Trumble SJ, Noren SR, Cornick LA, Hawke TJ, Kanatous SB (2010) Age-related differences in skeletal muscle lipid profiles of Weddell seals: clues to developmental changes. J Exp Biol 213:1676–1684
Wheatley KE, Nichols PD, Hindell MA, Harcourt RG, Bradshaw CJA (2008) Differential mobilization of blubber fatty acids in lactating Weddell seals: evidence of selective use. Physiol Biochem Zool 81:651–662
Worthy GAJ, Lavigne DM (1983) Energetics of fasting and subsequent growth in weaned harp seal pups, Phoca groenlandica. Can J Zool 61:447–456
Worthy GAJ, Lavigne DM (1987) Mass loss, metabolic rate, and energy utilization by harp and gray seal pups during the postweaning fast. Physiol Zool 60:352–364
Acknowledgments
D. Crocker, P. Webb, S. Hayes, J. Burns, and many graduate and undergraduate students provided assistance in the field. The rangers and staff at Año Nuevo State Reserve, California State Park were invaluable to the completion of the project. J. Pettinger and A. Banks provided assistance in the laboratory at the University of California, Santa Cruz; and S. Lang, C. Beck and others provided assistance in the laboratory at Dalhousie University, Halifax, Nova Scotia. This project was supported by grants to D.P. Noren from the American Museum of Natural History (Lerner-Gray Fund for Marine Research), Friends of Long Marine Laboratory, and University of California Natural Reserve System (Mildred E. Mathias Graduate Student Research Grant). Further support was provided by National Science Foundation grants to T.M. Williams (OPP-9909862) and to D.P. Costa and M.E. Goebel (OPP-9500072). Grants to S.J. Iverson through the Natural Sciences and Engineering Research council (NSERC) of Canada provided additional support. D.P. Noren was supported in part by NSF grant 9730462 to H. Kibak and D.P. Costa and by a Graduate Assistance in Areas of National Need Fellowship, Dept. of Ecology and Evolutionary Biology, University of California, Santa Cruz (UCSC). A portion of this work was performed while D.P. Noren was a National Research Council Postdoctoral Research Associate at the National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Seattle, Washington. All procedures comply with the current laws of the United States of America. Research was approved by the Chancellor’s Animal Research Committee (UCSC) and conducted under U.S. National Marine Fisheries Service permit 836. Two anonymous reviewers provided constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Noren, D.P., Budge, S.M., Iverson, S.J. et al. Characterization of blubber fatty acid signatures in northern elephant seals (Mirounga angustirostris) over the postweaning fast. J Comp Physiol B 183, 1065–1074 (2013). https://doi.org/10.1007/s00360-013-0773-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-013-0773-0