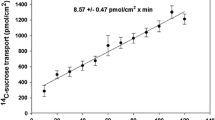
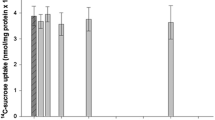
Abstract
Purified epithelial brush border membrane vesicles (BBMV) were produced from the hepatopancreas of the Atlantic White shrimp, Litopeneaus setiferus, using standard methods originally developed for mammalian tissues and previously applied to other crustacean and echinoderm epithelia. These vesicles were used to study the cation dependency of sugar and amino acid transport across luminal membranes of hepatopancreatic epithelial cells. 3H-d-glucose uptake by BBMV against transient sugar concentration gradients occurred when either transmembrane sodium or potassium gradients were the only driving forces for sugar accumulation, suggesting the presence of a possible coupled transport system capable of using either cation. 3H-l-histidine transport was only stimulated by a transmembrane potassium gradient, while 3H-l-leucine uptake was enhanced by either a sodium or potassium gradient. These responses suggest the possible presence of a potassium-dependent transporter that accommodates either amino acid and a sodium-dependent system restricted only to l-leucine. Uptake of 3H-l-leucine was significantly stimulated (P < 0.05) by several metallic cations (e.g., Zn2+, Cu2+, Mn2+, Cd2+, or Co2+) at external pH values of 7.0 or 5.0 (internal pH 7.0), suggesting a potential synergistic role of the cations in the transmembrane transfer of amino acids. 3H-l-histidine influxes (15 suptakes) were hyperbolic functions of external [zinc] or [manganese], following Michaelis–Menten kinetics. The apparent affinity constant (e.g., K m) for manganese was an order of magnitude smaller (K m = 0.22 μM Mn) than that for zinc (K m = 1.80 μM Zn), while no significant difference (P > 0.05) occurred between their maximal transport velocities (e.g., J max). These results suggest that a number of cation-dependent nutrient transport systems occur on the shrimp brush border membrane and aid in the absorption of these important dietary elements.





Similar content being viewed by others
References
Ahearn GA (1974) Kinetic characteristics of glycine transport by the isolated midgut of the marine shrimp, Penaeus marginatus. J Exp Biol 61:677–696
Ahearn GA (1976) Co-transport of glycine and sodium across the mucosal border of the midgut epithelium in the shrimp, Penaeus marginatus. J Physiol 258:499–520
Ahearn GA, Grover ML, Dunn RE (1985) Glucose transport by lobster hepatopancreatic brush border membrane vesicles. Am J Physiol 248:R133–R141
Ahearn GA, Behnke RD (1991) l-proline transport systems of starfish pyloric caeca. J Exp Biol 158:477–493
Ahearn GA, Gerencser GA, Thamotharan M, Behnke RD, Lemme TH (1992) Invertebrate gut diverticula are nutrient absorptive organs. Am J Physiol 263:R472–R481
Ahearn GA, Maginniss LA (1977) Kinetics of glucose transport by the perfused mid-gut of the freshwater prawn, Macrobrachium rosenbegii. J Physiol (Lond) 271:319–336
Biber J, Stieger B, Haase W, Murer H (1981) A high yield preparation for rat kidney brush border membranes. Different behavior of lysosomal markers. Biochim Biophys Acta 647:169–176
Bifano TD, Alegria TGP, Terra WR (2010) Transporters involved in glucose and water absorption in the Dysdercus peruvianus (Hemiptera: Pyrrhocoridae) anterior midgut. Comp Biochem Physiol B 157:1–9
Blaya JA, Vazquez CM, Muriana FJ, Ruiz-Gutierrez V, Bolufer J (1998) Alpha-MeGlc and d-glucose transport by hepatopancreatic brush border membrane vesicles from prawn. J Physiol Biochem 54:1–7
Castagna M, Shayakul C, Trotti D, Sacchi VF, Harvey WR, Hediger MA (1998) Cloning and characrterization of a potassium-coupled amino acid transporter. Proc Natl Acad Sci USA 95:5395–5400
Conrad EM, Ahearn GA (2005) 3H-l-histidine and 65Zn2+ are co-transported by a dipeptide transport system in lobster (Homarus americanus) intestine. J Exp Biol 208:287–296
Conrad EM, Ahearn GA (2007) Transepithelial transport of zinc and l-histidine across perfused intestine of American lobster, Homarus americanus. J Comp Physiol B 177:297–307
Forcella M, Berra E, Giacchini R, Parenti P (2006) Leucine transport in brush border membrane vesicles from freshwater insect larvae. Arch Insect Biochem Physiol 63:100–122
Giordana B, Leonardi MG, Casartelli M, Consonni P, Parent P (1998) K+-neutral amino acid symport of Bombyx mori larval midgut: a system operative in extreme conditions. Am J Physiol 274:R1361–R1371
Glover CN, Bury NR, Hogstrand C (2003) Zinc uptake across the apical membrane of freshwater rainbow trout intestine is mediated by high affinity, low affinity, and histidine-facilitated pathways. Biochim Biophys Acta 1614:211–219
Glover CN, Wood CM (2008a) Absorption of copper and copper-histidine complexes across the apical surface of freshwater rainbow trout intestine. J Comp Physiol B 178:101–109
Glover CN, Wood CM (2008b) Histidine absorption across apical surfaces of freshwater rainbow trout intestine: mechanistic characterization and the influence of copper. J Membr Biol 221:87–95
Goldner AM, Schultz SG, Curran PF (1969) Sodium and sugar fluxes across the mucosal border of rabbit ileum. J Gen Physiol 53:362–383
Hennigan BB, Wolfersberger MG, Parthasarathy R, Harvey WR (1993a) Cation-dependent leucine, alanine, and phenylalanine uptake at pH 10 in brush-border membrane vesicles from larval Manduca sexta midgut. Biochim Biophys Acta 1148:209–215
Hennigan BB, Wolfersberger MG, Harvey WR (1993b) Neutral amino acid symport in larval Manduca sexta midgut brush-border membrane vesicles deduced from cation-dependent uptake of leucine, alanine, and phenylalanine. Biochim Biophys Acta 1148:216–222
Hopfer U, Nelson K, Perrotto J, Isselbacher KJ (1973) Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248:25–32
Horn NM, Thomas AL, Tompkins JD (1995) The effect of histidine and cysteine on zinc influx into rat and human erythrocytes. J Physiol 489:73–80
Horn NM, Thomas AL (1996) Interactions between the histidine stimulation of cadmium and zinc influx into human erythrocytes. J Physiol 496:711–718
Kessler M, Acuto O, Storelli C, Murer H, Semenza G (1978) A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of d-glucose and choline transport systems. Biochim Biophys Acta 506:136–154
Mullins A, Ahearn GA (2008) Zinc dependent l-leucine uptake in Homarus americanus mid-gut. 4th CPB Meeting in Africa: Mara 2008. In: Morris S, Vosloo A (eds) Molecules to migration: the pressures of life. Medimond Publishing Co., Italy, pp 83–90
Obi I, Sterling KM, Ahearn GA (2011) Transepithelial d-glucose and d-fructose transport across the American lobster, Homarus americanus, intestine. J Exp Biol 214:2337–2344
Parenti P, Forcella M, Pugliese A, Giacchini R, Rossar B, Hanozet GM (2001) Leucine transport in membrane vesicles from Chironomus riparius larvae displays a mélange of Crown-group features. Arch Insect Biochem Physiol 48:51–62
Reuveni M, Dunn PE (1993) Absorption pathways of amino acids in the midgut of Manduca sexta larvae. Insect Biochem Mol Biol 23:959–966
Stevens BR, Ross HJ, Wright EM (1982) Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J Membr Biol 66:213–225
Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y (1999) Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 274:19745–19751
Tacnet F, Watkins DW, Ripoche P (1990) Studies of zinc transport into brush-border membrane vesicles isolated from pig small intestine. Biochim Biophys Acta 1024:323–330
Tacnet F, Lauthier F, Ripoche P (1993) Mechanisms of zinc transport into pig small intestine brush-border membrane vesicles. J Physiol 465:57–72
Verri T, Mandal A, Zilli L, Bossa D, Mandal PK, Ingrosso L, Zonno V, Vilella S, Ahearn GA, Storelli C (2001) d-glucose transport in decapod crustacean hepatopancreas. Comp Biochem Physiol A 130:585–606
Vilella S, Zonno V, Ingrosso L, Verri T, Storelli C (1998) Electroneutral Na+/H+ exchange in brush-border membrane vesicles from Penaeus japonicas hepatopancreas. Am J Physiol 274:R486–R493
Vilella S, Zilli L, Ingrosso L, Schiavone R, Zonno V, Verri T, Storelli C (2003) Differential expression of Na+/d-glucose cotransport in isolated cells of Marsupenaeus japonicas hepatopancreas. J Comp Physiol B 173:679–686
Wright SH, Ahearn GA (1997) Nutrient absorption in invertebrates. In: Handbook of physiology (sect. 13: comparative physiology), vol II, Chapter 16, pp 1137–1206
Acknowledgments
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2010-65206-20617 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Simmons, T., Mozo, J., Wilson, J. et al. Cation-dependent nutrient transport in shrimp digestive tract. J Comp Physiol B 182, 209–216 (2012). https://doi.org/10.1007/s00360-011-0621-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0621-z