Abstract
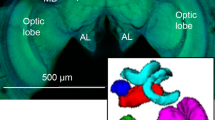
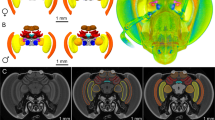
Alternative reproductive tactics (ARTs) occur when there is categorical variation in the reproductive strategies of a sex within a population. These different behavioral phenotypes can expose animals to distinct cognitive challenges, which may be addressed through neuroanatomical differentiation. The dramatic phenotypic plasticity underlying ARTs provides a powerful opportunity to study how intraspecific nervous system variation can support distinct cognitive abilities. We hypothesized that conspecific animals pursuing ARTs would exhibit dissimilar brain architecture. Dimorphic males of the bee species Centris pallida and Amegilla dawsoni use alternative mate location strategies that rely primarily on either olfaction (large-morph) or vision (small-morph) to find females. This variation in behavior led us to predict increased volumes of the brain regions supporting their primarily chemosensory or visual mate location strategies. Large-morph males relying mainly on olfaction had relatively larger antennal lobes and relatively smaller optic lobes than small-morph males relying primarily on visual cues. In both species, as relative volumes of the optic lobe increased, the relative volume of the antennal lobe decreased. In addition, A. dawsoni large males had relatively larger mushroom body lips, which process olfactory inputs. Our results suggest that the divergent behavioral strategies in ART systems can be associated with neuroanatomical differentiation.




Similar content being viewed by others
Availability of data and materials
Data archived in Dryad: https://doi.org/10.5061/dryad.bcc2fqzcd.
Code availability
Not applicable.
References
Aiello LC, Wheeler P (1995) The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthro 36:199–221
Alcock J (1976) The social organization of male populations of Centris pallida (Hymenoptera, Anthophoridae). Psyche 83:121–131
Alcock J (1979) The evolution of intraspecific diversity in male reproductive strategies in some bees and wasps. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic Press, New York, pp 381–402
Alcock J (1984) Long-term maintenance of size variation in populations of Centris pallida (Hymenoptera: Anthophoridae). Evol 38:220–223
Alcock J (1997) Competition from large males and the alternative mating tactics of small males of Dawson’s burrowing bee (Amegilla dawsoni) (Apidae, Apinae, Anthophorini). J Insect Behav 10:99–113
Alcock J (2013) Role of body size in the competition for mates by males of Centris pallida (Anthophorinae: Hymenoptera). Southwest Nat 58:427–430
Alcock J, Buchmann S (1985) The significance of the post-insemination display by male Centris pallida (Hymenoptera: Anthophoridae). Z Tierpsychol 68:231–243
Alcock J, Jones E, Buchmann S (1976) Location before emergence of the female bee, Centris pallida, by its male (Hymenoptera: Anthrophoridae). J Zool 179:189–199
Alcock J, Jones E, Buchmann S (1977) Male mating strategies in the bee Centris pallida Fox (Anthophoridae: Hymenoptera). Am Nat 111:145–155
Barton RA (1998) Visual specialization and brain evolution in primates. Proc R Soc Lond B Biol Sci 265:1933–1937
Barton RA, Harvey PH (2000) Mosaic evolution of brain structure in mammals. Nature 405:1055–1058
Barton RA, Purvis A, Harvey PH (1995) Evolutionary radiation of visual and olfactory systems in primates, bats and insectivores. Philos Trans R Soc L B Biol Sci 348:381–392
Bernstein S, Bernstein RA (1969) Relationship between foraging efficiency and the size of the head and component brain and sensory structures in the red wood ant. Brain Res 16:85–104
Brandstaetter AS, Bastin F, Sandoz JC (2014) Honeybee drones are attracted by groups of consexuals in a walking simulator. J Exp Biol 217:1278–1285
Bulova S, Purce K, Khodak P, Sulger E, O’Donnell S (2016) Into the black and back: the ecology of brain investment in Neotropical army ants (Formicidae: Dorylinae). Sci Nat 103:31
Chittka L, Niven J (2009) Are bigger brains better? Curr Biol 19:R995–R1008
Danforth B, Desjardins CA (1999) Male dimorphism in Perdita portalis (Hymenoptera, Andrenidae) has arisen from preexisting allometric patterns. Insectes Soc 46:18–28
Fahrbach SE (2006) Structure of the mushroom bodies of the insect brain. Ann Rev Entomol 51:209–232
Godfrey RK, Gronenberg W (2019) Brain evolution in social insects: advocating for the comparative approach. J Comp Physiol A 205:13–32
Gordon DG, Traniello JF (2018) Synaptic organization and division of labor in the exceptionally polymorphic ant Pheidole rhea. Neurosci Lett 676:46–50
GraphPad Software (2018) GraphPad Prism v 8.3.0 for Windows. La Jolla
Gronenberg W, Hölldobler B (1999) Morphologic representation of visual and antennal information in the ant brain. J Comp Neurol 412:229–240
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Healy S, Guilford T (1990) Olfactory-bulb size and nocturnality in birds. Evolution 44:339–346
Houston TF (1991) Ecology and behaviour of the bee Amegilla (Asaropoda) dawsoni (Rayment) with notes on a related species (Hymenoptera: Anthophoridae). Rec West Aust Mus 15:591–609
Keesey IW, Grabe V, Gruber L, Koerte S, Obiero GF, Bolton G, Khallaf MA, Kunert G, Lavista-Llanos S, Valenzano DR, Rybak J, Barrett BA, Knaden M, Hansson BS (2019) Inverse resource allocation between vision and olfaction across the genus Drosophila. Nat Commun 10:1162
Kenyon FC (1896) The brain of the bee. A preliminary contribution to the morphology of the nervous system of the Arthropoda. J Comp Neurol 6:133–210
Kotrschal K, van Staaden MJ, Huber R (1998) Fish brains: evolution and environmental relationships. Rev Fish Biol Fish 8:373–408
Kukuk PF (1996) Male dimorphism in Lasioglossum (Chilalictus) hemichalceum: the role of larval nutrition. J Kans Entomol Soc 69:147–157
Liao WB, Lou SL, Zeng Y, Kotrschal A (2016) Large brains, small guts: the expensive tissue hypothesis supported with anurans. Am Nat 188:693–700
Luo Y, Zhong MJ, Huang Y, Li F, Liao WB, Kotrschal A (2017) Seasonality and brain size are negatively associated in frogs: evidence for the expensive brain framework. Sci Rep 7:16629
Montgomery SH, Ott SR (2015) Brain composition in Godyris zavaleta, a diurnal butterfly, reflects an increased reliance on olfactory information. J Comp Neurol 523:869–891
Niven JE (2016) Neuronal energy consumption: biophysics efficiency and evolution. Curr Opin Neurobiol 41:129–135
Niven JE, Laughlin SB (2008) Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol 211:1792–1804
O’Donnell S, Clifford MR, DeLeon S, Papa C, Zahedi N, Bulova SJ (2013) Brain size and visual environment predict species differences in paper wasp sensory processing brain regions (Hymenoptera: Vesidae, Polistinae). Brain Behav Evol 82:177–184
O’Donnell S, Bulova SJ, Barrett M, Fiocca K (2017) Size constraints and sensory adaptations affect mosaic brain evolution in paper wasps (Vespidae: Epiponini). Biol J Linn Soc 123:302–310
O’Donnell S, Bulova S, Barrett M, von Beeren C (2018) Brain investment under colony-level selection: soldier-specialization in Eciton army ants (Formicidae: Dorylinae). BMC Zool 3:3
Oliveira RF, Taborsky M, Brockmann HJ (2008) Alternative reproductive tactics: an integrative approach. Cambridge University Press, Cambridge
Özer I, Carle T (2020) Back to the light, coevolution between vision and olfaction in the “dark-flies” (Drosophila melanogaster). PLoS ONE 15:e0228939
Paulk AC, Gronenberg W (2008) Higher order visual input to the mushroom bodies in the bee, Bombus impatiens. Arthropod Struct Dev 37:443–458
Paxton RJ (2005) Male mating behaviour and mating systems of bees: an overview. Apidologie 36:145–156
Rehan SM, Bulova SJ, O’Donnell S (2015) Cumulative effects of foraging behavior and social dominance on brain development in a facultatively social bee (Ceratina australensis). Brain Behav Evol 85:117–124
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sheehan ZBV, Kamhi JF, Seid MA, Narendra A (2019) Differential investment in brain regions for a diurnal and nocturnal lifestyle in Australian Myrmecia ants. J Comp Neurol 527:1261–1277
Sherry DF (2006) Neuroecology. Ann Rev Psychol 57:167–197
Shuster SM (2010) Alternative mating strategies. In: Fox C, Westneat DF (eds) Evolutionary behavioral ecology. Cambridge University Press, Cambridge
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Simmons LW, Alcock J, Reeder A (2003) The role of cuticular hydrocarbons in male attraction and repulsion by female Dawson’s burrowing bee, Amegilla dawsoni. Anim Behav 66:677–685
Slater GP, Yocum GD, Bowsher JH (2020) Diet quantity influences caste determination in honeybees (Apis mellifera). Proc R Soc Lond B Biol Sci 287:20200614
Snelling RR (1984) Studies on the taxonomy and distribution of American Centridine bees (Hymenoptera: Anthophoridae). Contrib Sci 347:1–69
Soares D, Niemiller ML (2013) Sensory adaptations of fishes to subterranean environments. Bioscience 63:274–283
Stieb SM, Kelber C, Wehner R, Rossler W (2011) Antennal-lobe organization in desert ants of the genus Cataglyphis. Brain Behav Evol 77:136–146
Stöckl A, Heinze S, Charalabidis A, el Jundi B, Warrant E, Kelber A (2016) Differential investment in visual and olfactory brain areas reflects behavioral choices in hawk moths. Sci Rep 6:26041
Tierney SM, Friedrich M, Humphreys WF, Jones TM, Warrant EJ, Wcislo WT (2017) Consequences of evolutionary transitions in changing photic environments. Aust Entomol 56:23–46
van der Woude E, Groothius J, Smid HM (2019) No gains for bigger brains: functional and neuroanatomical consequences of relative brain size in a parasitic wasp. J Evol Biol 32:694–705
Warrant EJ, Locket NA (2004) Vision in the deep sea. Biol Rev Camb Philos Soc 79:671–712
Wenner AM (1974) Information transfer in honeybees: a population approach. In: Kramer L, Pliver P, Alloway R (eds) Advances in the study of communication and affect. Plenum Press, New York, pp 133–169
Wilczynski W (1984) Central nervous systems subserving a homoplasous periphery. Am Zool 24:755–763
Acknowledgements
The authors would like to thank Dan Papaj, Antionette “Toni” Roe, Kay Richter, and Kit Prendergast for field assistance; Karmi Oxman, Stefan Bonestroo, Virginia Caponera, Christian Cabuslay, Rhe Congdon, Devneet Kainth, and Cheyenne McNair for laboratory assistance; Nikolai Tatarnic and the Western Australian Museum (Perth, Australia) for export permits from Australia to the USA; and John Alcock, Bruce Taubert, and Leigh Simmons for nesting site information. RA support for MRB from Drexel College of Arts and Sciences. Buchmann acknowledges support for A. dawsoni specimen collection and preparation from National Science Foundation Grant number 1929499, collection and export permits obtained through the Australian Government Department of the Environment in collaboration with the Western Australia Museum (AU027; Buchmann: US174). Species are not endangered nor protected.
Funding
RA support to MRB from the Drexel College of Arts and Sciences. SB received support from National Science Foundation Grant number 1929499 to collect and prepare A. dawsoni specimen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrett, M., Schneider, S., Sachdeva, P. et al. Neuroanatomical differentiation associated with alternative reproductive tactics in male arid land bees, Centris pallida and Amegilla dawsoni. J Comp Physiol A 207, 497–504 (2021). https://doi.org/10.1007/s00359-021-01492-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-021-01492-4