Abstract
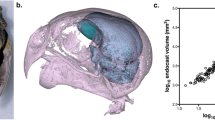
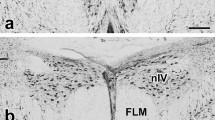
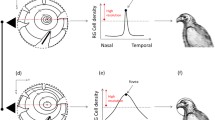
Eye morphology and the retinal topography of animals that live in either ‘open’ (e.g., grassland) or ‘enclosed’ (e.g., forest) terrestrial habitats show common adaptations to constraints imposed by these different habitat types. Although relationships between habitat and the visual system are well documented in most vertebrates, relatively few studies have examined this relationship in birds. Here, we compare eye shape and retinal topography across seven species from the family Phasianidae (Galliformes) that are diurnally active in either open or enclosed habitats. Species from enclosed habitats have significantly larger corneal diameters, relative to transverse diameters, than species from open habitats, which we predict serves to enhance visual sensitivity. Retinal topography, however, was similar across all seven species and consisted of a centrally positioned area centralis and a weak horizontal visual streak, with no discernible fovea. In the Japanese quail (Coturnix japonica), there was also a dorso-temporal extension of increased neuron density and, in some specimens, a putative area dorsalis. The total number of neurons in the retinal ganglion cell layer was correlated with retinal whole-mount area. Average and peak neuron densities were similar across species, with the exception of the Japanese quail, which had greater average and peak densities. Peak anatomical spatial resolving power was also similar among species, ranging from approximately 10–13 cycles/°. Overall, the pattern of retinal topography we found in phasianids is associated with ground-foraging in birds and presumably facilitates the identification of small food items on the ground as well as other visually guided behaviors, irrespective of habitat type.




Similar content being viewed by others
References
Ahnelt PK, Schubert C, Kübber-Heiss A, Schiviz A, Anger E (2006) Independent variation of retinal S and M cone photoreceptor topographies: a survey of four families of mammals. Vis Neurosci 23:429–435
Appleby MC, Mench JA, Hughes BO (2004) Poultry behaviour and welfare. CABI Publishing, Wallingford
Archibald HL (1976) Spring drumming patterns of ruffed grouse. Auk 93:808–829
Bailes HJ, Trezise AEO, Collin SP (2006) The number, morphology, and distribution of retinal ganglion cells and optic axons in the Australian lungfish Neoceratodus forsteri (Krefft 1870). Vis Neurosci 23:257–273
Bergerud AT, Gratson MW (eds) (1988) Adaptive strategies and population ecology of northern grouse. In: Theory and synthesis, vol 2. University of Minnesota Press, Minneapolis
Binggeli RL, Paule WJ (1969) The pigeon retina: quantitative aspects of optic nerve and ganglion cell layer. J Comp Neurol 137:1–18
Bischof H-J (1988) The visual field and visually guided behavior in the zebra finch (Taeniopygia guttata). J Comp Physiol A 163:329–337
Boire D, Dufour JS, Theoret H, Ptito M (2001) Quantitative analysis of the retinal ganglion cell layer in the ostrich, Struthio camelus. Brain Behav Evol 58:343–355
Bravo H, Pettigrew JD (1981) The distribution of neurons projecting from the retina and visual cortex to the thalamus and tectum opticum of the barn owl, Tyto alba, and burrowing owl, Speotyto cunicularia. J Comp Neurol 199:419–441
Budnik V, Mpodozis J, Varela FJ, Maturana HR (1984) Regional specialization of the quail retina: ganglion cell density and oil droplet distribution. Neurosci Lett 51:145–150
Chen Y, Naito J (1999) A quantitative analysis of the cells in the ganglion cell layer of the chick retina. Brain Behav Evol 53:75–86
Christisen GC (1970) The chukar partridge: its introduction, life history and management. Biological Bulletin No. 4, Nevada Department of Fish and Game, Reno
Coimbra JP, Trévia N, Marceliano ML, da Silveira Andrade-Da-Costa BL, Picanço-Diniz CW, Yamada ES (2009) Number and distribution of neurons in the retinal ganglion cell layer in relation to foraging behaviors of tyrant flycatchers. J Comp Neurol 514:66–73
Collin SP (1999) Behavioural ecology and retinal cell topography. In: Archer SN, Djamgoz MBS, Loew ER, Partridge JC, Vellarga S (eds) Adaptive mechanisms in the ecology of vision. Kluwer, Dordrecht, pp 509–535
Collin SP (2008) A web-based archive for topographic maps of retinal cell distribution in vertebrates. Clin Exp Optom 91:85–95
Collin SP, Pettigrew JD (1988a) Retinal topography in reef teleosts. I. Some species with well-developed areae but poorly-developed streaks. Brain Behav Evol 31:269–282
Collin SP, Pettigrew JD (1988b) Retinal topography in reef teleosts. II. Some species with prominent horizontal streaks and high-density areae. Brain Behav Evol 31:283–295
Collin SP, Pettigrew JD (1988c) Retinal ganglion cell topography in teleosts: a comparison between Nissl-stained material and retrograde labelling from the optic nerve. J Comp Neurol 276:412–422
Collin SP, Pettigrew JD (1989) Quantitative comparison of the limits on visual spatial resolution set by the ganglion cell layer in twelve species of reef teleosts. Brain Behav Evol 34:184–192
Collin SP, Hoskins RV, Partridge JC (1998) Seven retinal specializations in the tubular eye of the deep-sea pearleye, Scopelarchus michaelsarsi: a case study in visual optimization. Brain Behav Evol 51:291–314
Corfield JR, Gsell AC, Brunton D, Heesy CP, Hall MI, Acosta ML, Iwaniuk AI (2011) Anatomical specializations for nocturnality in a critically endangered parrot, the kakapo (Strigops habroptilus). PLoS One 6:e22945
Crowe TM, Bowie RCK, Blommer P, Mandiwana TG, Hedderson TAJ, Randi E, Pereira SL, Wakeling J (2006) Phylogenetics, biogeography and classification of, and character evolution in, gamebirds (Aves: Galliformes): effects of character exclusion, data partitioning and missing data. Cladistics 22:495–532
del Hoyo J, Elliott A, Sargatal J (eds) (1994) Handbook of the birds of the world. New world vultures to guineafowl, vol 2. Lynx Edicions, Barcelona
Dolan T, Fernández-Juricic E (2010) Retinal ganglion cell topography of five species of ground-foraging birds. Brain Behav Evol 75:111–121
Ehrlich D (1981) Regional specialization of the chick retina as revealed by the size and density of neurons in the ganglion cell layer. J Comp Neurol 195:643–657
Ehrlich D, Morgan IG (1980) Kainic acid destroys displaced amacrine cells in post-hatch chicken retina. Neurosci Lett 17:43–48
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Fernández-Juricic E, Moore BA, Doppler M, Freeman J, Blackwell BF, Lima SL, DeVault TL (2011a) Testing the terrain hypothesis: Canada geese see their world laterally and obliquely. Brain Behav Evol 77:147–158
Fernández-Juricic E, Gall MD, Dolan T, O’Rourke C, Thomas S, Lynch JR (2011b) Visual systems and vigilance behaviour of two ground-foraging avian prey species: white-crowned sparrows and California towhees. Anim Behav 81:705–713
Fite KV, Rosenfield-Wessels S (1975) A comparative study of deep avian foveas. Brain Behav Evol 12:97–115
Foster RG, Korf H-W, Schalken JJ (1987) Immunocytochemical markers revealing retinal and pineal but not hypothalamic photoreceptor systems in the Japanese quail. Cell Tissue Res 248:161–167
Fowler J, Cohen L (1990) Practical statistics for field biology. Wiley, Chichester
Ghim MM, Hodos W (2006) Spatial contrast sensitivity of birds. J Comp Physiol A 192:523–534
Gullion GW (1965) Improvements in methods for trapping and marking ruffed grouse. J Wildl Manage 29:109–116
Gullion GW (1984) Grouse of the north shore. Willow Creek Press, Oshkosh
Gundersen HJG (1977) Notes on the estimation of the numerical density of arbitrary particles: the edge effect. J Microsc 111:219–223
Güntürkün O, Miceli D, Watanabe M (1993) Anatomy of the avian thalamofugal pathway. In: Zeigler HP, Bischof H-J (eds) Vision, brain, and behavior in birds. MIT Press, Cambridge, pp 115–135
Hall MI, Ross CF (2007) Eye shape and activity pattern in birds. J Zool 271:437–444
Hart NS (2002) Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol 205:3925–3935
Hayes BP (1984) Cell populations of the ganglion cell layer: displaced amacrine and matching amacrine cells in the pigeon retina. Exp Brain Res 56:565–573
Hayes BP, Brooke MD (1990) Retinal ganglion cell distribution and behavior in Procellariiform seabirds. Vision Res 30:1277–1289
Hayes BP, Holden AL (1983) The distribution of displaced ganglion cells in the retina of the pigeon. Exp Brain Res 49:181–188
Howard CV, Reed MG (2005) Unbiased stereology: three-dimensional measurement in microscopy, 2nd edn. BIOS Scientific Publishers, Abingdon
Hughes A (1975) A comparison of retinal ganglion cell topography in the plains and tree kangaroo. J Physiol (Lond) 244:61–63
Hughes A (1977) The topography of vision in mammals of contrasting lifestyles: comparative optics and retinal organization. In: Cresitelli F (ed) Handbook of sensory physiology, vol VIII/5. Springer, Berlin, pp 613–756
Hughes A (1981) One brush tailed possum can browse as much pasture as 0.06 sheep which may indicate why this “arboreal” animal has a visual streak: some comments on the “terrain” theory. Vis Res 21:957–958
Hughes A (1985) New perspectives in retinal organization. In: Osbourne NN, Chader G (eds) Progress in retinal research. Pergamon Press, New York, pp 243–313
Ikushima M, Watanabe M, Ito H (1986) Distribution and morphology of retinal ganglion cells in the Japanese quail. Brain Res 376:320–334
Inzunza O, Bravo H, Smith RL, Angel M (1991) Topography and morphology of retinal ganglion cells in Falconiforms: a study on predatory and carrion-eating birds. Anat Rec 229:271–277
Iwaniuk AN, Heesy CP, Hall MI (2010) Morphometrics of the eyes and orbits of the nocturnal swallow-tailed gull (Creagrus furcatus). Can J Zool 88:855–865
Johnsgard PA (1973) Grouse and quails of North America. University of Nebraska, Lincoln
Kiltie RA (2000) Scaling of visual acuity with body size in mammals and birds. Funct Ecol 2000(14):226–234
Kirk EC (2004) Comparative morphology of the eye in primates. Anat Rec 281A:1095–1103
Kirk EC (2006) Eye morphology in cathemeral lemurids and other mammals. Folia Primatol (Basel) 77:27–49
Kolm N, Stein RW, Mooers AØ, Verspoor JJ, Cunningham JA (2006) Can sexual selection drive female life histories? A comparative study on Galliform birds. J Evol Biol 20:627–638
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lisney TJ, Collin SP (2008) Retinal ganglion cell distribution and spatial resolving power in elasmobranchs. Brain Behav Evol 72:59–77
Lisney TJ, Iwaniuk AN, Bandet MV, Wylie DW (2012) Eye shape and retinal topography in owls (Aves: Strigiformes). Brain Behav Evol 79:218–236
Martin GR (1982) An owl’s eye: schematic optics and visual performance in Strix aluco L. J Comp Physiol A 145:341–349
Martin GR (1994) Form and function in the optical structure of bird eyes. In: Green PR (ed) Davies MNO. Springer, Berlin, pp 5–34
Meyer DB (1977) The avian eye and its adaptations. In: Cresitelli F (ed) Handbook of sensory physiology, vol VIII/5. Springer, Berlin, pp 549–611
Meyer DB, May HC Jr (1973) The topographic distribution of rods and cones in the adult chicken retina. Exp Eye Res 17:347–355
Moroney MK, Pettigrew JD (1987) Some observations on the visual optics of kingfishers (Aves, Coraciformes, Alcedinidae). J Comp Physiol A 160:137–149
Morris VB (1982) An afoveate area centralis in the chick retina. J Comp Neurol 210:198–203
Necker R (2007) Head-bobbing of walking birds. J Comp Physiol A 193:1177–1183
Oehme H (1961) Vergleichend-histologische Untersuchungen an der Retina von Eulen. Zool Jb Anat 79:439–478 (Article in German)
Ovington JD, Madgwick HAI (1955) A comparison of light in different woodlands. Forestry 28:141–146
Peterson EH, Ulinski PS (1979) Quantitative studies of retinal ganglion cells in a turtle, Pseudemys scripta elegans. 1. Number and distribution of ganglion cells. J Comp Neurol 186:17–42
Pettigrew JD, Manger PR (2008) Retinal ganglion cell density of the black rhinoceros (Diceros bicornis ): calculating visual resolution. Vis Neurosci 25:215–220
Pettigrew JD, Dreher B, Hopkins CS, McCall MJ, Brown M (1988) Peak density and distribution of ganglion cells in the retinae of microchiropteran bats: implications for visual acuity. Brain Behav Evol 32:39–56
Pettigrew JD, Bhagwandin A, Haagensen M, Manger PR (2010) Visual acuity and heterogeneities of retinal ganglion cell densities and the tapetum lucidum of the African elephant (Loxodonta africana). Brain Behav Evol 75:251–261
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rahman ML, Sugita S, Aoyama M, Sugita S (2006) Number, distribution and size of retinal ganglion cells in the jungle crow (Corvus macrorhynchos). Anat Sci Int 81:253–259
Rahman ML, Aoyama M, Sugita S (2007) Regional specialization of the tree sparrow Passer montanus retina: ganglion cell density and oil droplet distribution. Ornithol Sci 6:95–105
Rasband WS (1997–2011) Image J. US National Institutes of Health, Bethesda
Reymond L (1985) Spatial visual acuity of the eagle Aquila audax: a behavioural, optical and anatomical investigation. Vis Res 25:1477–1491
Reymond L (1987) Spatial visual acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vis Res 27:1859–1874
Rojas LM, Ramirez Y, McNeil R, Mitchell M, Marin G (2004) Retinal morphology and electrophysiology of two Caprimulgiformes birds: the cave-living and nocturnal oilbird (Steatornis caripensis), and the crepuscularly and nocturnally foraging common pauraque (Nyctidromus albicollis). Brain Behav Evol 64:19–33
Rounsley KJ, McFadden SA (2005) Limits of visual acuity in the frontal field of the rock pigeon (Columba livia). Perception 34:983–993
Schaeffel F, Howland HC (1989) Visual optics in normal and ametropic chickens. Clin Vision Sci 3:83–98
Scheaffer RL, Mendenhall W, Ott L (1996) Elementary survey sampling, 5th edn. PWS-Kent, Boston
Schiviz AN, Ruf T, Kuebber-Heiss A, Schubert C, Ahnelt PK (2008) Retinal cone topography of artiodactyl mammals: influence of body height and habitat. J Comp Neurol 507:1336–1350
Schlee MA (1983) An experimental study of prey-attack behavior in the European blackbird Turdus m. merula L. Z Tierpsychol 61:203–224
Schmitz C, Hof PR (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroant 20:93–114
Schmitz L, Wainwright PC (2011) Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evol Biol 11:338
Snyder AW, Miller WH (1977) Photoreceptor diameter and spacing for highest resolving power. J Opt Soc Am 67:696–698
Stone J (1981) The wholemount handbook: a guide to the preparation and analysis of retinal wholemounts. Clarendon Press, Sydney
Straznicky C, Chehade M (1987) The formation of the area centralis of the retinal ganglion cell layer of the chick. Development 100:411–420
Ullmann JFP, Moore BA, Temple SH, Fernández-Juricic E, Collin SP (2012) The retinal wholemount technique: a window to understanding the brain and behaviour. Brain Behav Evol 79:26–44
Veilleux CC, Lewis RJ (2011) Effects of habitat light intensity on mammalian eye shape. Anat Rec 294:905–914
Walls GL (1942) The vertebrate eye and its adaptive radiation. Cranbrook Institute of Science, Bloomfield Hills
Warrant E (2004) Vision in the dimmest habitats on Earth. J Comp Physiol A 190:765–789
Wathey JC, Pettigrew JD (1989) Quantitative analysis of the retinal ganglion cell layer and optic nerve of the barn owl Tyto alba. Brain Behav Evol 33:279–292
Wood CA (1917) The fundus oculi of birds, especially as viewed by the ophthalmoscope: a study in comparative anatomy and physiology. Lakeside Press, Chicago
Zeigler HP, Bischof H-J (eds) (1993) Vision, brain, and behavior in birds. MIT Press, Cambridge
Zeigler HP, Jäger R, Palacios AG (1993) Sensorimotor mechanisms and pecking in the pigeon. In: Zeigler HP, Bischof H-J (eds) Vision, brain, and behavior in birds. MIT Press, Cambridge, pp 265–283
Acknowledgments
This study was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants to DRW and ANI. We wish to thank Bill Young, Jodi Saucier, Greg Sanbourne, George Iwaniuk and Udo Hannebaum for providing specimens for our study and Maxime Garcia for assistance in trapping ruffed grouse. The grouse were collected under Alberta Department of Sustainable Resource Development Research and Collection Permits 47079 and 47080. All of our methods adhered to Canadian Council for Animal Care Guidelines and were approved by the University of Lethbridge Animal Welfare Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lisney, T.J., Iwaniuk, A.N., Kolominsky, J. et al. Interspecifc variation in eye shape and retinal topography in seven species of galliform bird (Aves: Galliformes: Phasianidae). J Comp Physiol A 198, 717–731 (2012). https://doi.org/10.1007/s00359-012-0742-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-012-0742-1