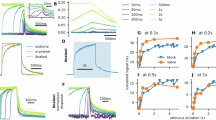
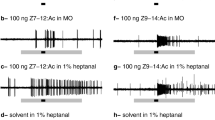
Abstract
Odor source localization is an important animal behavior. Male moths locate mates by tracking sex pheromone emitted by conspecific females. During this type of behavior, males exhibit a combination of upwind surge and zigzagging flight. Similarly, the male walking moth Bombyx mori responds to transient pheromone exposure with a surge in movement, followed by sustained zigzagging walking. The initial surge direction is known to be influenced by the pheromone input pattern. Here, we identified the sensory input patterns that determine the initial walking direction of males. We first quantified the stimulus by measuring electroantennogram values, which were used as a reference for subsequent tests. We used a brief stimulus pulse to examine the relationship between sensory stimulus patterns and the turning direction of initial surge. We found that the difference in input timing and intensity between left and right antennae affected the walking direction, indicating that B. mori integrate bilateral pheromone information during orientation behavior. When we tested pheromone stimulation for longer periods, turning behavior was suppressed, which was induced by stimulus cessation. This study contributes toward understanding efficient strategies for odor-source localization that is utilized by walking insects.





Similar content being viewed by others
References
Agarwal G, Isacoff E (2011) Specializations of a pheromonal glomerulus in the Drosophila olfactory system. J Neurophysiol 105:1711–1721
Ai H, Kanzaki R (2004) Modular organization of the silkmoth antennal lobe macroglomerular complex revealed by voltage-sensitive dye imaging. J Exp Biol 207:633–644
Baker TC (1990) Upwind flight and casting flight: complementary and tonic systems used for location of sex pheromone sources by male moths. In: Døving KB (ed) proceedings of the tenth international symposium on olfaction and taste GCS A/S, Oslo, Norway, pp18–25
Baker TC, Haynes KF (1987) Manoeuvres used by flying male oriental fruit moths to relocate a sex pheromone plume in an experimentally shifted wind-field. Phsyiol Entomol 12:263–279
Baker TC, Kuenen LPS (1982) Pheromone source location by flying moths: A supplementary non-anemotactic mechanism. Science 216:424–426
Baker TC, Willis MA, Phelan PL (1984) Optomotor anemotaxis polarizes self-steered zigzagging in flying moths. Physiol Entomol 9:365–376
Bell WJ, Tobin TR (1981) Orientation to sex pheromone in the American cockroach: analysis of chemo-orientation mechanisms. J Insect Physiol 27:501–508
Borst A, Heisenberg M (1982) Osmotropotaxis in Drosophila melanogaster. J Comp Phsyiol A 147:479–484
Burgstaller M, Tichy H (2011) Functional asymmetries in cockroach ON and OFF olfactory receptor neurons. J Neurophyiol 105:834–845
Butenandt VA, Beckmann R, Stamm D, Hecker E (1959) Über den Sexual-Lockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z Naturforsch 14b:283–284
Cardé RT, Willis MA (2008) Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol 34:854–866
David CT, Kennedy JS, Ludlow AR (1983) Finding of a sex pheromone source by gypsy moths released in the field. Nature 303:804–806
Duistermars BJ, Chow DM, Frye MA (2009) Flies require bilateral sensory input to track odor gradients in flight. Curr Biol 19:1301–1307
Farkas SR, Shorey HH (1972) Chemical trail-following by flying insects: a mechanism for orientation to a distant odor source. Science 178:67–68
Gardiner JM, Atema J (2010) The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr Biol 20:1187–1191
Heinze S, Gotthardt S, Homberg U (2009) Transformation of polarized light information in the central complex of the locust. J Neurosci 29:11783–11793
Iwano M, Hill ES, Mori A, Mishima T, Mishima T, Ito K, Kanzaki R (2010) Neurons associated with the flip-flop activity in the lateral accessory lobe and the ventral protocerebrum of the silkworm moth brain. J Comp Neurol 518:366–388
Johnsen PB, Teeter JH (1980) Spatial gradient detection of chemical cues by catfish. J Comp Physiol A 140:95–99
Kaissling K-E (1971) Insect olfaction. In: Beidler LM (ed) Handbook of sensory physiology IV. Springer-Verlag, Berlin, pp 351–431
Kanzaki R (1996) Behavioral and neural basis of instinctive behavior in insects: odor-source searching strategies without memory and learning. Robot Auton Syst 18:33–43
Kanzaki R (1998) Coordination of wing motion and walking suggests common control of zigzag motor program in a male silkworm moth. J Comp Physiol A 182:267–276
Kanzaki R, Mishima T (1996) Pheromone-triggered ‘flipflopping’ neural signals correlate with activities of neck motor neurons of a male moth, Bombyx mori. Zool Sci 13:79–87
Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG (1989) Physiology and morphology of projection neurons in the antennal lobe of the male moth Manduca sexta. J Comp Physiol A 165:427–453
Kanzaki R, Arbas EA, Hildebrand JG (1991) Physiology and morphology of descending neurons in pheromone-processing olfactory pathways in the male moth, Manduca sexta. J Comp Physiol A 169:1–14
Kanzaki R, Sugi N, Shibuya T (1992) Self-generated zigzag turning of Bombyx mori males during pheromone-mediated upwind walking. Zool Sci 9:515–516
Kanzaki R, Ikeda S, Shibuya T (1994) Morphological and physiological properties of pheromone-induced flipflopping descending interneurons of the male silkworm moth, Bombyx mori. J Comp Physiol A 175:1–14
Kanzaki R, Soo K, Seki Y, Wada S (2003) Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem Senses 28:113–130
Kennedy JS (1940) The visual responses of flying mosquitoes. Proc Zool Soc London 109:221–242
Kennedy JS (1983) Zigzagging and casting as a programmed response to wind-borne odour: a review. Physiol Entomol 8:109–120
Kennedy JS, Marsh D (1974) Pheromone-regulated anemotaxis in flying moths. Science 184:999–1001
Kent KS, Harrow ID, Quartararo P, Hildebrand JG (1986) An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res 245:237–245
Kojima T, Sakuma M, Fukui M, Kuwahara Y (2003) Spatial orientation of the mould mite, Tyrophagus putrescentiae (Schrank) (Acarina: Acaridae), in the computer-programmed olfactory field. J Acarol Soc Jpn 12:93–102
Koontz MA, Schneider D (1987) Sexual dimorphism in neuronal projections from the antennae of the silk moths (Bombyx mori, Antheraea polyphemus) and gypsy moth (Lymantria dispar). Cell Tissue Res 249:39–50
Kramer E (1975) Orientation of the amle silkmoth to the sex attractant bombykol. In: Denton DA, Coghlan JP (eds) Olfaction and taste V. Academic Press, New York, pp 329–335
Kramer E (1986) Turbulent diffusion and pheromone-triggered anemotaxis. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Oxford University Press, Oxford, pp 59–67
Kuenen LPS, Baker TC (1982) A non-anemotactic mechanism used in pheromone source location by flying moths. Physiol Entomol 7:277–289
Kuenen LPS, Cardé RT (1994) Strategies for recontacting a lost pheromone plume: casting and upwind flight in the male gypsy moth. Physiol Entomol 19:15–29
Lei H, Anton S, Hansson BS (2001) Olfactory protocerebral pathways processing sex pheromone and plant odor information in the male moth Agrotis segetum. J Comp Neurol 432:356–370
Lei H, Riffell JA, Gage SL, Hildebrand JG (2009) Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol 8:21
Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB (2008) Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci 11:187–199
Mafra-Neto A, Cardé RT (1994) Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature 369:142–144
Mafra-Neto A, Cardé RT (1995) Influence of plume structure and pheromone concentration on upwind flight of Cadra cautella males. Physiol Entomol 20:117–133
Mishima T, Kanzaki R (1998) Coordination of flipflopping neural signals and head turning during pheromone-mediated walking in a male silkworm moth Bombyx mori. J Comp Physiol A 183:273–282
Mishima T, Kanzaki R (1999) Physiological and morphological characterization of olfactory descending interneurons of the male silkworm moth. Zool Sci 184:143–160
Murlis J, Jones CD (1981) Fine-scale structure of odour plumes in relation to insect orientation to distant pheromone and other attractant sources. Physiol Entomol 6:71–86
Murlis J, Elkinton JS, Cardé RT (1992) Odor plumes and how insects use them. Annu Rev Entomol 37:505–532
Namiki S, Kanzaki R (2005) Pheromonal processing pathways in the silkmoth brain. Zool Sci Abstract 22:1484
Namiki S, Kanzaki R (2011a) Offset response of the olfactory projection neurons in the moth antennal lobe. BioSystems 103:348–354
Namiki S, Kanzaki R (2011b) Heterogeneity in dendritic morphology of moth antennal lobe projection neurons. J Comp Neurol 519:3367–3386
Namiki S, Iwabuchi S, Kanzaki R (2008) Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J Comp Physiol A 194:501–515
Obara Y (1979) Bombyx mori mating dance: an essential in locating the female. Appl Entomol Zool 14:130–132
Olberg RM (1983) Interneurons sensitive to female pheromone in the deutocerebrum of the male silkworm moth, Bombyx mori. Physiol Entomol 8:419–428
Ono T (1980) Role of the scales as a releaser of the copulation attempt in the silkworm moth, Bombyx mori (Lepidoptera, Bombycidae). Kontyu Tokyo 48:540–544
Porter J, Craven B, Khan RM, Chang SJ, Kang I, Judkewitz B, Volpe J, Settles G, Sobel N (2007) Mechanisms of scent-tracking in humans. Nat Neurosci 10:27–29
Rajan R, Clement JP, Bhalla US (2006) Rats smell in stereo. Science 311:666–670
Ritzmann RE, Ridgel AL, Pollack AJ (2008) Multi-unit recording of antennal mechano-sensitive units in the central complex of the cockroach, Blaberus discoidalis. J Comp Physiol A 194:341–360
Sakurai T, Mitsuno H, Haupt SS, Uchino K, Yokohari F, Nishioka T, Kobayashi I, Sezutsu H, Tamura T, Kanzaki R (2011) A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genetics 7:e1002115
Seki Y, Aonuma H, Kanzaki R (2005) Pheromone processing center in the protocerebrum of Bombyx mori revealed by NO-induced anti-cGMP immunocytochemistry. J Comp Neruol 481:340–351
Steck K, Knaden M, Hansson BS (2010) Do desert ants smell the scenery in stereo? Animal Behav 79:939–945
Tichy H, Hinterwirth A, Gingl E (2005) Olfactory receptors on the cockroach antenna signal odour ON and odour OFF by excitation. Eur J Neurosci 22:3147–3160
Vergassola M, Villermaux E, Shraiman BI (2007) ‘Infotaxis’ as a strategy for searching without gradients. Nature 445:406–409
Vickers NJ (2000) Mechanisms of animal navigation in odor plumes. Biol Bull 198:203–212
Vickers NJ, Baker TC (1992) Male Heliothis virescens maintain upwind flight in response to experimentally pulsed filaments of their sex pheromone (Lepidoptera: Noctuidae). J Insect Behav 5:669–687
Vickers NJ, Baker TC (1994) Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc Natl Acad Sci USA 91:5756–5760
Vickers NJ, Baker TC (1996) Latencies of behavioral response to interception of filaments of sex pheromone and clean air influence flight track shape in Heliothis virescens (F.) males. J Comp Physiol A 178:831–847
Vickers NJ, Christensen TA, Baker TC, Hildebrand JG (2001) Odour-plume dynamics influence the brain’s olfactory code. Nature 410:466–470
Wada S, Kanzaki R (2005) Neural control mechanisms of the pheromone-triggered programmed behavior in male silkmoths revealed by double-labeling of descending interneurons and the motor neuron. J Comp Neurol 484:168–182
Webster DR, Rahman S, Dasi LP (2001) On the usefulness of bilateral comparison to tracking turbulent chemical odor plumes. Limnol Oceanogr 46:1048–1053
Willis MA, Arbas EA (1991) Odor-modulated upwind flight of sphinx moth, Manduca sexta L. J Comp Physiol A 169:427–440
Willis MA, Avondet JL (2005) Odor-modulated orientation in walking male cockroaches Periplaneta americana, and the effects of odor plumes of different structure. J Exp Biol 208:721–735
Willis MA, Baker TC (1987) Comparison of manoeuvres used by walking versus flying Grapholita molesta males during pheromone-mediated upwind movement. J Exp Biol 33:875–883
Acknowledgments
We are grateful to Prof. Dr. Shigeru Matsuyama (Graduate School of Life and Environmental Sciences, University of Tsukuba) for providing purified bombykol. This research was supported by: Research and Development of the Next-Generation Integrated Simulation of Living Matter, part of the Development and Use of the Next-Generation Supercomputer Project; a Grant-in-Aid for Scientific Research (B) (18370028) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); and a Sasagawa Research Grant from the Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
359_2011_708_MOESM1_ESM.tif
Supplementary Figure. 1. Visualization of airflow. Airflow from the stimulus cartridge visualized by TiCl4 is shown. The flow rate was 500 ml/min. The movie was acquired at 30 Hz. Relative intensity change was calculated and shown in pseudocolor. The flow was straight and the diameter of airflow at the position of several centimeters from the cartridge was approximately the same as that of the stimulus cartridge. (TIFF 2629 kb)
Rights and permissions
About this article
Cite this article
Takasaki, T., Namiki, S. & Kanzaki, R. Use of bilateral information to determine the walking direction during orientation to a pheromone source in the silkmoth Bombyx mori . J Comp Physiol A 198, 295–307 (2012). https://doi.org/10.1007/s00359-011-0708-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0708-8