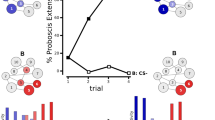
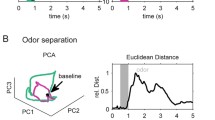
Abstract
Odors elicit spatio-temporal patterns of activity in the olfactory bulb of vertebrates and the antennal lobe of insects. There have been several reports of changes in these patterns following olfactory learning. These studies pose a conundrum: how can an animal learn to efficiently respond to a particular odor with an adequate response, if its primary representation already changes during this process? In this study, we offer a possible solution for this problem. We measured odor-evoked calcium responses in a subpopulation of uniglomerular AL output neurons in honeybees. We show that their responses to odors are remarkably resistant to plasticity following a variety of appetitive olfactory learning paradigms. There was no significant difference in the changes of odor-evoked activity between single and multiple trial forward or backward conditioning, differential conditioning, or unrewarded successive odor stimulation. In a behavioral learning experiment we show that these neurons are necessary for conditioned odor responses. We conclude that these uniglomerular projection neurons are necessary for reliable odor coding and are not modified by learning in this paradigm. The role that other projection neurons play in olfactory learning remains to be investigated.













Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- AL:
-
Antennal lobe
- CR:
-
Conditioned response
- CS−:
-
Non-rewarded stimulus
- CS+:
-
Rewarded (conditioned) stimulus
- ITI:
-
Inter-trial interval
- l-ACT:
-
Lateral antenno-cerebralis tract
- LP:
-
Lateral protocerebrum
- m-ACT:
-
Medial antenno-cerebralis tract
- MB:
-
Mushroom body
- ml-ACT:
-
Mediolateral antenno-cerebralis tract
- OB:
-
Olfactory bulb
- OSN:
-
Olfactory sensory neuron
- PN:
-
Projection neuron
- RM ANOVA:
-
Repeated measures analysis of variance
- SEG:
-
Subesophageal ganglion
- T1–T4:
-
Tracts 1–4 in the antennal lobe
- US:
-
Unconditioned stimulus
- PER:
-
Proboscis extension reflex
- VUMmx1:
-
Ventral unpaired neuron # 1 of the maxillary neuromere
References
Abel R (1997) Das olfaktorische System der Honigbiene: elektrophysiologische und morphologische Charakterisierung von Antennallobus Neuronen und deren beteiligung beim olfaktorischen Lernen. PhD Thesis, FU Berlin
Abel R, Rybak J, Menzel R (2001) Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol 437:363–383
Anton S, Hansson BS (1994) Central processing of sex pheromone, host odour, and oviposition deterrent information by interneurons in the antennal lobe of female Spodoptera littoralis (Lepidoptera: Noctuidae). J Comp Neurol 350:199–214
Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G (2005) Fast odor learning improves reliability of odor responses in the locust antennal lobe. Neuron 46:483–492
Bitterman ME, Menzel R, Fietz A, Schafer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119
Brennan PA, Schellinck HM, de la RC, Kendrick KM, Keverne EB (1998) Changes in neurotransmitter release in the main olfactory bulb following an olfactory conditioning procedure in mice. Neuroscience 87:583–590
Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG (2004) Learning modulates the ensemble representations for odors in primary olfactory networks. Proc Natl Acad Sci USA 101:10476–10481
Davis RL (2004) Olfactory learning. Neuron 44:31–48
Deisig N, Lachnit H, Giurfa M, Hellstern F (2001) Configural olfactory learning in honeybees: negative and positive patterning discrimination. Learn Mem 8:70–78
Ditzen M, Evers JF, Galizia CG (2003) Odor similarity does not influence the time needed for odor processing. Chem Senses 28:781–789
Ernst KD, Boeckh J, Boeckh V (1977) A neuroanatomical study on the organization of the central antennal pathways in insects. Cell Tissue Res 176:285–306
Faber T, Menzel R (2001) Visualizing mushroom body response to a conditioned odor in honeybees. Naturwissenschaften 88:472–476
Faber T, Joerges J, Menzel R (1999) Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci 2:74–78
Flanagan D, Mercer AR (1989) An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol 18:145–159
Friedrich RW (2002) Real time odor representations. Trends Neurosci 25:487–489
Galán RF, Weidert M, Menzel R, Herz A, Galizia CG (2006) Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput 18:10–25
Galizia CG, Menzel R (2001) The role of glomeruli in the neural representation of odours: results from optical recording studies. J Insect Physiol 47:115–130
Galizia CG, Vetter R (2004) Optical methods for analyzing odor-evoked activity in the insect brain. In: Christensen TA (ed) Advances in insect sensory neuroscience. CRC Press, Boca Raton, pp 349–392
Galizia CG, Joerges J, Kuttner A, Faber T, Menzel R (1997) A semi-in-vivo preparation for optical recording of the insect brain. J Neurosci Methods 76:61–69
Galizia CG, McIlwrath SL, Menzel R (1999a) A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res 295:383–394
Galizia CG, Sachse S, Rappert A, Menzel R (1999b) The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat Neurosci 2:473–478
Gerber B, Menzel R (2000) Contextual modulation of memory consolidation. Learn Mem 7:151–158
Giurfa M (2003). Cognitive neuroethology: dissecting non-elemental learning in a honeybee brain. Curr Opin Neurobiol 13:726–735
Gluck MA, Granger R (1993) Computational models of the neural bases of learning and memory. Annu Rev Neurosci 16:667–706
Guerrieri F, Schubert M, Sandoz JC, Giurfa M (2005) Perceptual and neural olfactory similarity in honeybees. PLoS Biol 3:e60
Hammer M (1993) An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366:59–63
Hammer M, Menzel R (1995) Learning and memory in the honeybee. J Neurosci 15:1617–1630
Hammer M, Menzel R (1998) Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem 5:146–156
Haupt SS (2004) Antennal sucrose perception in the honey bee (Apis mellifera L.): behaviour and electrophysiology. J Comp Physiol A 190:735–745
Heisenberg M (2003) Mushroom body memoir: from maps to models. Nat Rev Neurosci 4:266–275
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Johnson BA, Woo CC, Duong H, Nguyen V, Leon M (1995) A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res 699:192–200
Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG (1989) Physiology and morphology of projection neurons in the antennal lobe of the male moth Manduca sexta. J Comp Physiol A 165:427–453
Kreissl S, Eichmuller S, Bicker G, Rapus J, Eckert M (1994) Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honeybee. J Comp Neurol 348:583–595
Linster C, Johnson BA, Morse A, Yue E, Leon M (2002) Spontaneous versus reinforced olfactory discriminations. J Neurosci 22:6842–6845
Lohmann C, Wong RO (2005) Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium 37:403–409
Lu XC, Slotnick BM (1998) Olfaction in rats with extensive lesions of the olfactory bulbs: implications for odor coding. Neuroscience 84:849–866
Malun D (1991) Synaptic relationships between GABA-immunoreactive neurons and an identified uniglomerular projection neuron in the antennal lobe of Periplaneta americana: a double-labeling electron microscopic study. Histochemistry 96:197–207
Malun D, Giurfa M, Galizia CG, Plath N, Brandt R, Gerber B, Eisermann B (2002) Hydroxyurea-induced partial mushroom body ablation does not affect acquisition and retention of olfactory differential conditioning in honeybees. J Neurobiol 53:343–360
Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L (2002) Representation of the glomerular olfactory map in the Drosophila brain. Cell 109:243–255
Mauelshagen J (1993) Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J Neurophysiol 69:609–625
Menzel R (1990) Learning, memory, and “cognition” in honey bees. In: Kesner RP, Olton DS (eds) Neurobiology of comparative cognition. Erlbaum Inc, Hillsdale, pp 237–292
Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol A 185:323–340
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5:62–71
Menzel R, Muller U (1996) Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci 19:379–404
Menzel R, Hammer M, Braun G, Mauelshagen J (1991) Neurobiology of learning and memory in honeybees. In: Goodmann LJ, Fisher RC (eds) The behaviour and physiology of bees. CAB International, Wallingford, pp 323–353
Mobbs PG (1982) The brain of the honeybee Apis mellifera I. The connections and spatial organization of the mushroom bodies. Philos Trans R Soc Lond B 298:309–354
Mori K, Nagao H, Yoshihara Y (1999) The olfactory bulb: coding and processing of odor molecule information. Science 286:711–715
Müller D (2000a) Die olfaktorische Kodierung: Plastizität und Stabilität—Eine elektrophysiologische Analyse der Ausgangsneurone des Antennallobus der Honigbiene. PhD Thesis, FU Berlin
Müller U (2000b) Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron 27:159–168
Müller D, Gerber B, Hellstern F, Hammer M, Menzel R (2000) Sensory preconditioning in honeybees. J Exp Biol 203:1351–1364
Müller D, Abel R, Brandt R, Zockler M, Menzel R (2002) Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A 188:359–370
Mutoh H, Yuan Q, Knopfel T (2005) Long-term depression at olfactory nerve synapses. J Neurosci 25:4252–4259
Nishino H, Yamashita S, Yamazaki Y, Nishikawa M, Yokohari F, Mizunami M (2003) Projection neurons originating from thermo- and hygrosensory glomeruli in the antennal lobe of the cockroach. J Comp Neurol 455:40–55
Sachse S, Galizia CG (2002) Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol 87:1106–1117
Sachse S, Rappert A, Galizia CG (1999) The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci 11:3970–3982
Sandoz JC, Menzel R (2001) Side-specificity of olfactory learning in the honeybee: generalization between odors and sides. Learn Mem 8:286–294
Sandoz JC, Galizia CG, Menzel R (2003) Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honeybee antennal lobes. Neuroscience 120:1137–1148
Stocker RF, Lienhard MC, Borst A, Fischbach KF (1990) Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 262:9–34
Stocker RF, Heimbeck G, Gendre N, de Belle JS (1997) Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol 32:443–456
Stopfer M, Laurent G (1999) Short-term memory in olfactory network dynamics. Nature 402:664–668
Sullivan RM, Wilson DA (1995) Dissociation of behavioral and neural correlates of early associative learning. Dev Psychobiol 28:213–219
Szyszka P (2005) Odor coding and neural plasticity in the mushroom body of the honeybee. PhD Thesis, FU Berlin
Tanaka NK, Awasaki T, Shimada T, Ito K (2004) Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol 14:449–457
Tully T, Preat T, Boynton SC, Del Vecchio M (1994) Genetic dissection of consolidated memory in Drosophila. Cell 79:35–47
Wilson DA, Stevenson RJ (2003) Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci Biobehav Rev 27:307–328
Winnington AP, Napper RM, Mercer AR (1996) Structural plasticity of identified glomeruli in the antennal lobes of the adult worker honey bee. J Comp Neurol 365:479–490
Wong AM, Wang JW, Axel R (2002) Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109:229–241
Woo CC, Coopersmith R, Leon M (1987) Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol 263:113–125
Yokohari F (1983) The coelocapitular sensillum, an antennal hygro- and thermoreceptive sensillum of the honey bee, Apis mellifera L. Cell Tissue Res 233:355–365
Yokohari F, Tominaga Y, Tateda H (1982) Antennal hygroreceptors of the honey bee, k. Cell Tissue Res 226:63–73
Yokoi M, Mori K, Nakanishi S (1995) Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92:3371–3375
Yu D, Ponomarev A, Davis RL (2004) Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron 42:437–449
Acknowledgments
Support from Volkswagenstiftung, Human Frontier Science Program, Deutscher Akademischer Austauschdienst and Deutsche Forschungsgemeinschaft SFB 515 is acknowledged. Thanks also to Uli Müller for intellectual exchange during the entire project, to Paul Szyszka for valuable discussion and to Silke Sachse, Daniela Pelz and the referees for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peele, P., Ditzen, M., Menzel, R. et al. Appetitive odor learning does not change olfactory coding in a subpopulation of honeybee antennal lobe neurons. J Comp Physiol A 192, 1083–1103 (2006). https://doi.org/10.1007/s00359-006-0152-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0152-3