Abstract
Purpose
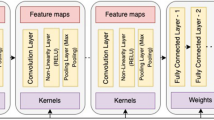
As computational power has improved over the past 20 years, the daily application of machine learning methods has become more prevalent in daily life. Additionally, there is increasing interest in the clinical application of machine learning techniques. We sought to review the current literature regarding machine learning applications for patient-specific urologic surgical care.
Methods
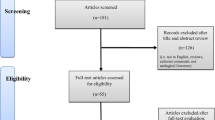
We performed a broad search of the current literature via the PubMed-Medline and Google Scholar databases up to Dec 2020. The search terms “urologic surgery” as well as “artificial intelligence”, “machine learning”, “neural network”, and “automation” were used.
Results
The focus of machine learning applications for patient counseling is disease-specific. For stone disease, multiple studies focused on the prediction of stone-free rate based on preoperative characteristics of clinical and imaging data. For kidney cancer, many studies focused on advanced imaging analysis to predict renal mass pathology preoperatively. Machine learning applications in prostate cancer could provide for treatment counseling as well as prediction of disease-specific outcomes. Furthermore, for bladder cancer, the reviewed studies focus on staging via imaging, to better counsel patients towards neoadjuvant chemotherapy. Additionally, there have been many efforts on automatically segmenting and matching preoperative imaging with intraoperative anatomy.
Conclusion
Machine learning techniques can be implemented to assist patient-centered surgical care and increase patient engagement within their decision-making processes. As data sets improve and expand, especially with the transition to large-scale EHR usage, these tools will improve in efficacy and be utilized more frequently.


Similar content being viewed by others
References
Beckmann JS, Lew D (2016) Reconciling evidence-based medicine and precision medicine in the era of big data: challenges and opportunities. Genome Med 8:134. https://doi.org/10.1186/s13073-016-0388-7
Yu K-H, Beam AL, Kohane IS (2018) Artificial intelligence in healthcare. Nat Biomed Eng 2:719–731. https://doi.org/10.1038/s41551-018-0305-z
Tran BX, Vu GT, Ha GH et al (2019) Global evolution of research in artificial intelligence in health and medicine: a bibliometric study. J Clin Med. https://doi.org/10.3390/jcm8030360
Moustris GP, Hiridis SC, Deliparaschos KM, Konstantinidis KM (2011) Evolution of autonomous and semi-autonomous robotic surgical systems: a review of the literature. Int J Med Robot Comput Assist Surg MRCAS 7:375–392. https://doi.org/10.1002/rcs.408
Roehrborn CG, Teplitsky S, Das AK (2019) Aquablation of the prostate: a review and update. Can J Urol 26:20–24
Rajkomar A, Dean J, Kohane I (2019) Machine learning in medicine. N Engl J Med 380:1347–1358. https://doi.org/10.1056/NEJMra1814259
Kriegeskorte N, Golan T (2019) Neural network models and deep learning. Curr Biol 29:R231–R236. https://doi.org/10.1016/j.cub.2019.02.034
Raman JD, Aditya B, Karim B et al (2010) Residual fragments after percutaneous nephrolithotomy: cost comparison of immediate second look flexible nephroscopy versus expectant management. J Urol 183:188–193. https://doi.org/10.1016/j.juro.2009.08.135
Aminsharifi A, Irani D, Pooyesh S et al (2017) Artificial neural network system to predict the postoperative outcome of percutaneous nephrolithotomy. J Endourol 31:461–467. https://doi.org/10.1089/end.2016.0791
Aminsharifi A, Irani D, Tayebi S et al (2020) Predicting the postoperative outcome of percutaneous nephrolithotomy with machine learning system: software validation and comparative analysis with Guy’s stone score and the CROES nomogram. J Endourol 34:692–699. https://doi.org/10.1089/end.2019.0475
Gomha MA, Sheir KZ, Showky S et al (2004) Can we improve the prediction of stone-free status after extracorporeal shock wave lithotripsy for ureteral stones? a neural network or a statistical model? J Urol 172:175–179. https://doi.org/10.1097/01.ju.0000128646.20349.27
Seckiner I, Seckiner S, Sen H et al (2017) A neural network—based algorithm for predicting stone-free status after ESWL therapy. Int Braz J Urol Off J Braz Soc Urol 43:1110–1114. https://doi.org/10.1590/S1677-5538.IBJU.2016.0630
Osawa T, Hafez KS, Miller DC et al (2016) Comparison of percutaneous renal mass biopsy and R.E.N.A.L. nephrometry score nomograms for determining benign vs malignant disease and low-risk vs high-risk renal tumors. Urology 96:87–92. https://doi.org/10.1016/j.urology.2016.05.044
Kocak B, Kaya OK, Erdim C et al (2020) Artificial intelligence in renal mass characterization: a systematic review of methodologic items related to modeling, performance evaluation, clinical utility, and transparency. AJR Am J Roentgenol 215:1113–1122. https://doi.org/10.2214/AJR.20.22847
Tanaka T, Huang Y, Marukawa Y et al (2020) Differentiation of small (≤ 4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. Am J Roentgenol 214:605–612. https://doi.org/10.2214/AJR.19.22074
Yu H, Scalera J, Khalid M et al (2017) Texture analysis as a radiomic marker for differentiating renal tumors. Abdom Radiol 42:2470–2478. https://doi.org/10.1007/s00261-017-1144-1
Auffenberg GB, Ghani KR, Ramani S et al (2019) askMUSIC: leveraging a clinical registry to develop a new machine learning model to inform patients of prostate cancer treatments chosen by similar men. Eur Urol 75:901–907. https://doi.org/10.1016/j.eururo.2018.09.050
Zupan B, Demsar J, Kattan MW et al (2000) Machine learning for survival analysis: a case study on recurrence of prostate cancer. Artif Intell Med 20:59–75. https://doi.org/10.1016/s0933-3657(00)00053-1
Wong NC, Lam C, Patterson L, Shayegan B (2019) Use of machine learning to predict early biochemical recurrence after robot-assisted prostatectomy. BJU Int 123:51–57. https://doi.org/10.1111/bju.14477
Garapati SS, Hadjiiski L, Cha KH et al (2017) Urinary bladder cancer staging in CT urography using machine learning. Med Phys 44:5814–5823. https://doi.org/10.1002/mp.12510
Liu J, Wang S, Turkbey EB et al (2015) Computer-aided detection of renal calculi from noncontrast CT images using TV-flow and MSER features. Med Phys 42:144–153. https://doi.org/10.1118/1.4903056
Liu J, Wang S, Linguraru MG et al (2015) Computer-aided detection of exophytic renal lesions on non-contrast CT images. Med Image Anal 19:15–29. https://doi.org/10.1016/j.media.2014.07.005
Iglesias JE, Sabuncu MR (2015) Multi-atlas segmentation of biomedical images: a survey. Med Image Anal 24:205–219. https://doi.org/10.1016/j.media.2015.06.012
Huo Y, Braxton V, Herrell SD et al (2017) Automated characterization of pyelocalyceal anatomy using CT urograms to aid in management of kidney stones. In: Cardoso MJ, Arbel T, Luo X et al (eds) Computer assisted and robotic endoscopy and clinical image-based procedures. Springer, pp 99–107
Wang H, Suh JW, Das SR et al (2013) Multi-atlas segmentation with joint label fusion. IEEE Trans Pattern Anal Mach Intell 35:611–623. https://doi.org/10.1109/TPAMI.2012.143
Nosrati MS, Amir-Khalili A, Peyrat J-M et al (2016) Endoscopic scene labelling and augmentation using intraoperative pulsatile motion and colour appearance cues with preoperative anatomical priors. Int J Comput Assist Radiol Surg 11:1409–1418. https://doi.org/10.1007/s11548-015-1331-x
Kavoussi NL, Pitt B, Ferguson JM et al (2020) Accuracy of touch-based registration during robotic image-guided partial nephrectomy before and after tumor resection in validated phantoms. J Endourol. https://doi.org/10.1089/end.2020.0363
Svoboda E (2019) Your robot surgeon will see you now. Nature 573:S110–S111. https://doi.org/10.1038/d41586-019-02874-0
Ghani KR, Yunfan L, Hei L et al (2017) Pd46-04 video analysis of skill and technique (vast): machine learning to assess surgeons performing robotic prostatectomy. J Urol 197:e891–e891. https://doi.org/10.1016/j.juro.2017.02.2376
Hung AJ, Chen J, Che Z et al (2018) Utilizing machine learning and automated performance metrics to evaluate robot-assisted radical prostatectomy performance and predict outcomes. J Endourol 32:438–444. https://doi.org/10.1089/end.2018.0035
Hung AJ, Chen J, Ghodoussipour S et al (2019) A deep-learning model using automated performance metrics and clinical features to predict urinary continence recovery after robot-assisted radical prostatectomy. BJU Int 124:487–495. https://doi.org/10.1111/bju.14735
Kantarjian H, Yu PP (2015) Artificial intelligence, big data, and cancer. JAMA Oncol 1:573–574. https://doi.org/10.1001/jamaoncol.2015.1203
Ngiam KY, Khor IW (2019) Big data and machine learning algorithms for health-care delivery. Lancet Oncol 20:e262–e273. https://doi.org/10.1016/S1470-2045(19)30149-4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doyle, P.W., Kavoussi, N.L. Machine learning applications to enhance patient specific care for urologic surgery. World J Urol 40, 679–686 (2022). https://doi.org/10.1007/s00345-021-03738-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03738-x