Abstract
Purpose
To compare the ability of Prostate Health Index (PHI) to diagnose csPCa, with that of total PSA, PSA density (PSAD) and the multiparametric magnetic resonance (mpMRI) of the prostate.
Methods
We analysed a group of 395 men planned for a prostate biopsy who underwent a mpMRI of the prostate evaluated using the PIRADS v1 criteria. All patients had their PHI measured before prostate biopsy. In patients with an mpMRI suspicious lesions, an mpMRI/ultrasound software fusion-guided biopsy was performed first, with 12 core systematic biopsy performed in all patients. A ROC analysis was performed for PCa detection for total PSA, PSAD, PIRADS score and PHI; with an AUC curve calculated for all criteria and a combination of PIRADS score and PHI. Subsequent sub-analyses included patients undergoing first and repeat biopsy.
Results
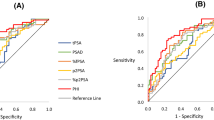
The AUC for predicting the presence of csPCa in all patients was 59.5 for total PSA, 69.7 for PHI, 64.9 for PSAD and 62.5 for PIRADS. In biopsy naive patients it was 61.6 for total PSA, 68.9 for PHI, 64.6 for PSAD and 63.1 for PIRADS. In patients with previous negative biopsy the AUC for total PSA, PHI, PSAD and PIRADS was 55.4, 71.2, 64.4 and 69.3, respectively. Adding of PHI to PIRADS increased significantly (p = 0.007) the accuracy for prediction of csPCa.
Conclusion
Prostate Health Index could serve as a tool in predicting csPCa. When compared to the mpMRI, it shows comparable results. The PHI cannot, however, help us guide prostate biopsies in any way, and its main use may, therefore, be in pre-MRI or pre-biopsy triage.

Similar content being viewed by others
References
Mottet N, van den Bergh RCN, Briers E et al (2018) EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2018. In: European Association of Urology Guidelines, 2018th edn. European Association of Urology Guidelines Office, Arnhem, The Netherlands
Shariat SF, Roehrborn CG (2008) Using biopsy to detect prostate cancer. Rev Urol 10:262–280
Shaw GL, Thomas BC, Dawson SN et al (2014) Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br J Cancer 110:2405–2411. https://doi.org/10.1038/bjc.2014.192
Thompson JE, van Leeuwen PJ, Moses D et al (2016) The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol 195:1428–1435. https://doi.org/10.1016/j.juro.2015.10.140
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822. https://doi.org/10.1016/S0140-6736(16)32401-1
Loeb S, Catalona WJ (2014) The Prostate Health index: a new test for the detection of prostate cancer. Ther Adv Urol 6:74–77. https://doi.org/10.1177/1756287213513488
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757. https://doi.org/10.1007/s00330-011-2377-y
Le BV, Griffin CR, Loeb S et al (2010) [-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J Urol 183:1355–1359. https://doi.org/10.1016/j.juro.2009.12.056
Catalona WJ, Partin AW, Sanda MG et al (2011) A Multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0–10.0 ng/ml prostate specific antigen range. J Urol 185:1650–1655. https://doi.org/10.1016/j.juro.2010.12.032
Filella X, Giménez N (2013) Evaluation of [-2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med 51:729–739. https://doi.org/10.1515/cclm-2012-0410
Loeb S, Shin SS, Broyles DL et al (2017) Prostate Health Index improves multivariable risk prediction of aggressive prostate cancer. BJU Int 120:61–68. https://doi.org/10.1111/bju.13676
White J, Shenoy BV, Tutrone RF et al (2018) Clinical utility of the Prostate Health Index (phi) for biopsy decision management in a large group urology practice setting. Prostate Cancer Prostatic Dis 21:78–84. https://doi.org/10.1038/s41391-017-0008-7
Rosenkrantz AB, Ginocchio LA, Cornfeld D et al (2016) Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: a multicenter study of six experienced prostate radiologists. Radiology 280:793–804. https://doi.org/10.1148/radiol.2016152542
Zhao C, Gao G, Fang D et al (2016) The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging 40:885–888. https://doi.org/10.1016/j.clinimag.2016.04.010
Druskin SC, Tosoian JJ, Young A et al (2018) Combining Prostate Health Index density, magnetic resonance imaging and prior negative biopsy status to improve the detection of clinically significant prostate cancer. BJU Int 121:619–626. https://doi.org/10.1111/bju.14098
Drost F-JH, Osses DF, Nieboer D et al (2019) Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012663.pub2
Schoots IG, Roobol MJ, Nieboer D et al (2015) Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 68:438–450. https://doi.org/10.1016/j.eururo.2014.11.037
Fütterer JJ, Briganti A, De Visschere P et al (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68:1045–1053. https://doi.org/10.1016/j.eururo.2015.01.013
Matoso A, Epstein JI (2019) Defining clinically significant prostate cancer on the basis of pathological findings. Histopathology 74:135–145. https://doi.org/10.1111/his.13712
Shah ZK, Elias SN, Abaza R et al (2015) Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol 22:467–474. https://doi.org/10.1016/j.acra.2014.11.007
Park BK, Kim B, Kim CK et al (2007) Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr 31:534–538. https://doi.org/10.1097/01.rct.0000250108.85799.e1
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol 69:16–40. https://doi.org/10.1016/j.eururo.2015.08.052
Auer T, Edlinger M, Bektic J et al (2017) Performance of PI-RADS version 1 versus version 2 regarding the relation with histopathological results. World J Urol 35:687–693. https://doi.org/10.1007/s00345-016-1920-5
Becker AS, Cornelius A, Reiner CS et al (2017) Direct comparison of PI-RADS version 2 and version 1 regarding interreader agreement and diagnostic accuracy for the detection of clinically significant prostate cancer. Eur J Radiol 94:58–63. https://doi.org/10.1016/j.ejrad.2017.07.016
Krishna S, McInnes M, Lim C et al (2017) Comparison of prostate imaging reporting and data system versions 1 and 2 for the detection of peripheral zone gleason Score 3 + 4 = 7 Cancers. Am J Roentgenol 209:W365–W373. https://doi.org/10.2214/AJR.17.17964
Gaur S, Harmon S, Mehralivand S et al (2018) Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 48:1326–1335. https://doi.org/10.1002/jmri.26025
Simmons LAM, Kanthabalan A, Arya M et al (2018) Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J Urol 200:1227–1234. https://doi.org/10.1016/j.juro.2018.07.001
Vaché T, Bratan F, Mège-Lechevallier F et al (2014) Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology 272:446–455. https://doi.org/10.1148/radiol.14131584
Funding
Supported by the Ministry of Health of the Czech Republic, grant nr. 15-27047A.
Author information
Authors and Affiliations
Contributions
All authors have made a significant contribution to the findings and methods in the paper. All authors have read and approved the final draft. JS: Data collection or management, Data analysis, Manuscript writing and editing, VA: Data collection or management, ZM: Data collection or management, Critical revision of the article, NV: Data collection or management, OČ: Data collection or management, Critical revision of the article, FV: Data collection or management, DO: Data collection or management, SH: Data collection or management, ŠV: Project development, Data analysis, Manuscript writing and editing, Critical revision of the article, RZ: Project development, Critical revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. The study was performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of Motol University Hospital approved this study.
Informed consent
All data were analysed retrospectively. Patients were informed and consented prior to both magnetic resonance imaging and prostate biopsy.
Availability of data and material
Source data are available for review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stejskal, J., Adamcová, V., Záleský, M. et al. The predictive value of the prostate health index vs. multiparametric magnetic resonance imaging for prostate cancer diagnosis in prostate biopsy. World J Urol 39, 1889–1895 (2021). https://doi.org/10.1007/s00345-020-03397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03397-4