Abstract
We report here structural morphology and nonlinear behavior of pure and co-doped Zn0.90-xFe0.1MxO with (M = Cu, Ni and (x = 0.00, 0.10) and (0.00 ≤ y ≤ 0.20)) at different sintering temperatures (Ts = 850 and 1000 °C). It is found that the co-doping of ZnO by (Fe + Cu) or (Fe + Ni) up to 0.30 does not deform the well-known wurtzite structure of ZnO, as well as pure and 0.1 of Fe-doped ZnO. The SEM micrographs did not show any secondary phases at the boundaries of grains as compared to ZnO, the average grain size is decreased for Fe and (Fe + Cu) samples, while it is increased for (Fe + Ni) samples. The nonlinear coefficient α and breakdown field EB are generally increased by 0.1 of Fe addition, but they are shifted to lower values as Ts increases for all samples. Furthermore, they are gradually increased/decreased to higher/lower values for (Fe + Cu/Fe + Ni) samples up to 0.30 of co-doping content. The values of α and EB are increased from 30.06, 2115.38 V/cm for ZnO at 850 °C to 50.07, 5012 V/cm by (0.1Fe + 0.2Cu) co-doping, and from 23.53, 1956.52 V/cm to 45.58, 4750 V/cm at 1000 °C, while they are, respectively, decreased by (0.1Fe + 0.2Ni) to 13.19, 312 V/cm and 11.85, 172.42 V/cm. Similar behavior was generally obtained for nonlinear conductivity σL and height of potential barrier φB, whereas the vice is versa for the behavior of leakage current Jk and residual voltage Kr. Our results are discussed in terms of the comparative participation between the effects of co-doping of (Fe + Cu) and (Fe + Ni) to ZnO for supporting the potential barrier as compared to individual doping by Fe, Cu and Ni. This study perhaps recommended these samples for optoelectronic and ferromagnetic investigation after COVID-19 is over.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
N-type ZnO exhibits an upturn region in the J-E curves (field drop in the grains) due to its electrostatic potential barrier formed at the grain boundaries [1,2,3,4]. The density of donor is about 1018 cm−3, and the average resistivity of grains and grains boundaries is, respectively, 10 and 1011 (Ω.cm), and consequently, the current is limited between the grains by a very high resistive [5,6,7]. The thin insulating layers, consisted of ZnO successive grains during synthesis at high temperatures, are mainly responsible for the upturn behavior and therefore support the barrier formation at the ZnO homo-junctions [8,9,10,11].
The upturn region of ZnO is usually controlled by the composition and heat treatment through doping and charge transfer during sintering at high temperatures [1, 2, 12]. Therefore, ZnO with low amount of additives has been early used as surge protecting element in the circuits of power transmissions as a varistor to avoid the high voltage overloads through controlling their nonlinear coefficient (α) and breakdown field (EB) up to 80 and 5000 V/cm, respectively [13,14,15]. With this purpose, some devices such as battery powered and mobile appliances require protection of transient low dc voltage up to 10 V, while the other devices need fast response protection up to 70 V [16, 17]. Nevertheless, these factors create needs for new advantages of ZnO varistor to be working over a wide range of α and EB with fast response.
The conductivity of n-type ZnO is introduced through twice-fold ionizations of Zn excess ions which can be usually assigned as point defects [18,19,20,21]. These defects originally formed below the conduction band of ZnO in the form of oxygen vacancies and work as donor defects [22,23,24]. However, the localized states of band gap deplete the ZnO carriers and lead to the creation of double Schottky barriers which govern ZnO nonlinear constants [1, 25, 26].
Although ZnO with 3-d transition metals can be early considered as a very complex chemical dopant, there is a lack of data based on ZnO thermal stability, particularly when ZnO synthesis at high temperatures [12, 27,28,29]. However, some of the models such as ion migration and defects suggested that Zn interstitials atom may be formed in the depletion layer during heating and then frozen by cooling down to RT [30, 31]. It is also accepted that some of these defects are formed at the grain boundary in the form of donor-like positive and acceptor-like negative charges, which normally affecting the ZnO nonlinear behaviors [32].
Anyhow, further research is needed for satisfying the overload voltage requirements such as nonlinearity and thermal stability. Previously, the nonlinear behaviors of ZnO with individual doping by magnetic and nonmagnetic additives were investigated [33,34,35,36,37,38,39,40]. It is approved that the nonlinear parameters of ZnO could be improved by low amount of dopants such as Fe, Co and Mn, but they are decreased by Cu and completely depressed by Ni. Additionally, such parameters could be improved by co-doping to ZnO by decreasing the ionization energy of acceptors and donors [41, 42]. Unfortunately, an appropriate obtaining or preparing samples permits the oxidation or reduction of ion dopants, which might result in the formation of the possible secondary phases, especially for the nanoparticles synthesis. Moreover, the nanoparticles have depressed the potential barrier of ZnO, which may limit the use of ZnO for varistor applications.
On the other hand, ZnO can form partially filled d-states or f-states, and partially filled the states of unpaired electrons of unpaired spins, which support the room temperature ferromagnetism (RTFM). Recently, RTFM of ZnO can be also enhanced through co-doping with two different 3-d transition ions [43, 44]. For example, the electrical conductivity of Zn1-xCo0.05FexO decreases with the increase in Fe, but an induced weak RTFM could be obtained as Fe increases [45]. It is also approved that the origin of the observed RTFM in Zn1-x(Fe + Co)xO samples is different than that of Zn1-x(Fe + Cu)xO samples [46]. Interestingly, Zn1-x(Fe + Co)xO does not have a tendency to form the Fe–O–Co ferromagnetic cluster, and therefore the double-exchange mechanism is not effective. In contrast, Zn1-x(Fe + Cu)xO has a tendency to form the Fe–O-Cu ferromagnetic cluster of the double-exchange mechanism with the charge transfer between Fe and Cu. Nerveless, the nonlinear properties are not presented and discussed in detail.
Sedky et.al have presented the XRD and FTIR analysis of Zn1-x-yFexMyO samples (M = Cu, Ni) samples with various Ts to explain the impact of co-doping on the crystal structure and elastic constants of ZnO [47]. At present, structural morphology and I-V characteristics are done on the same batch of samples. Although the nonlinear parameters are decreased as Ts increases for all samples, they are increased/decreased by (Fe + Cu/Fe + Ni) additions. Furthermore, a comparison between the reported data of individual dopants such as Fe, Cu and Ni with those obtained by co-doing was well estimated. However, these results gave us more hooping for optimizing ZnO nonlinear parameters through strong participation of (Fe + Cu) as compared to (Fe + Ni) and also with those reported for the individual dopants, which highlight the present work.
2 Experimental details
Zn1-x-yFexMyO co-doped samples with (M = Cu, Ni) and (x = 0.00, 0.10 and 0.00 ≤ y ≤ 0.20) were synthesized by using conventional solid-state reaction method. The powders of ZnO and Fe2O3, CuO and NiO were thoroughly mixed into eight samples and calcined in air for 16 h at 950 °C. The resulting powder of each sample was divided into two parts and separately pressed into 16 pellets, eight pellets for each series as (x = 0.00, y = 0.00 for S1), (x = 0.10, y = 0.00 for S2), (x = 0.10, y = 0.05, M = Cu for S3), (x = 0.10, y = 0.10, M = Cu for S4), (x = 0.10, y = 0.20, M = Cu for S5), (x = 0.10, y = 0.05, M = Ni for S6), (x = 0.10, y = 0.10, M = Ni for S7) and (x = 0.10, y = 0.20, M = Ni for S8). The two series of pellets were separately sintered at 850 °C and 1000 °C for 16 h and then slowly cooled to room temperature. For simplicity, the samples called as (S1(850), (S2(850), (S3(850), (S4(850), (S5(850 °C) (S6(850), (S7(850), (S8(850) series I and (S1(1000) (S2(1000) (S3(1000) (S4(1000), (S5(1000) (S6(1000) (S7(1000), (S8(1000) for series II. Structural morphology of the samples was determined by scanning electron microscope (SEM) JSM 5400 LV. Finally and with Keithley electrometer, 5 kV dc power supply and digital multimeter, I-V characteristics at RT (~ 4 mm thick and ~ 10 mm diameter) were obtained. The samples between two copper electrodes were polished and sandwiched, and the current was measured against applied voltage with the help of silver paint.
3 Results and discussions
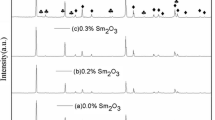
It is approved from the XRD patterns shown in Fig. 1a, b of the samples [47] that all identified diffraction peaks are belonging to the well-known hexagonal (wurtzite) structure of ZnO. There are some unknown peaks with relatively very low intensities formed at angles 2ϴ = 25.06°, 39.74°, 42.16° and 45.34° for series I (850 °C) and at 2ϴ = 42.28° and 45.04° for series II (1000 °C). Based on XRD data, one can say that the co-doping has a larger solubility within ZnO lattice and the limit of co-doping content could be extended up to 0.30 as compared to individual dopants [49,50,51,52].
The structural morphology of the samples shown in the SEM micrographs of Fig. 2a, b did not show any secondary phases over the matrix structure at the boundaries of grains for all samples. Most of the samples have cauliflower apparent shape for both series, and in contrast, some of them have a mountain stone-like shape with different sizes, especially at low Ts (850 °C). Generally, the grains of pure and Fe samples were composed of nearly homogeneous semi-spherical geometric like-shape, while in turn, the co-doped samples were composed of compact heterogeneous like-shapes of different sizes along with limited black regions between grains. It is also noted that the size of grains decreases as the Ts increases and it is greater for (Fe + Ni) samples as compared to the rest of the samples. Furthermore, the size of illuminating regions is greater which should as possible make them more conductive. The average grain size (D) is given by\(D = \frac{1.56\ell }{{MN}}\), \(\ell\) is the arbitrary length, M is the magnification of graph, and N is the counted grain boundaries [53]. The average grain size D against co-doping content is shown in Fig. 2c and also listed in Table 1. D is generally decreased by Fe, while it is increased by increasing Ts. As compared to Fe samples, more decrease/increase for (Fe + Cu)/(Fe + Ni) samples could be obtained. This behavior is related to the difference of ionic radii of the dopants as compared to Zn ion (Zn = 0.74 Å, Fe = 0.70 Å, Cu = 0.73 Å and Ni = 0.69 Å). However, a decrease in grain size for Fe and (Fe + Cu) samples as compared to (Fe + Ni) samples can be considered a good indicator that is reliable for their nonlinear property discussed in the next paragraph.
It is noted from the I-V curves shown in Fig. 3a–d that all samples show nonlinear behavior in the intermediate region between the first and third linear regions. Generally, the applied fields are shifted to lower values as Ts increases from 850 (series I) to 1000 °C (series II), whereas current densities are shifted to higher values. Similar behavior was obtained for (Fe + Ni) samples, while the vice is versa for the behaviors of the fields and currents for Fe and (Fe + Cu) samples.
To study the I-V behaviors in the ohmic regions as compared to the upturn regions for all samples, the breakdown field EB was obtained at 1 mA/cm2 of current density and also the leakage current Jk at the half of the breakdown field (EB/2) [54, 55]. The behaviors of EB and Jk against (Fe + M) and at different values of Ts are shown in Fig. 4a, b. It is evident from the figure that EB was decreased as Ts increases, while Jk was increased. As compared to pure ZnO, EB is gradually increased for Fe and (Fe + Cu) samples, but it is decreased for (Fe + Ni) samples, while the vice is versa for the behavior of Jk. These results indicated an inverse behavior between EB and Jk (EB α 1/Jk) for the considered samples. On the other hand, the residual voltage ratio Kr of ZnO is normally defined by the ratio between the field at a given current impulse to the EB for a fixed diameter and thickness. However, Kr has been obtained for ZnO in terms of the empirical relations between Kr and EB and between Kr and average grain size D as follows [56];
where b0, b1, c0, c1 and c2 are constants equal 1.21 V/cm, 1370 V/cm, 1.17, 0.069 (μm)−1 and 0.0013 (μm)−2 for ZnO, respectively. For simplicity, we have used similar relations for obtaining Kr for the present samples. The behavior of Kr against (Fe + M) and at different values of Ts is shown in Fig. 5 (a, b) for both series. Although the behavior of Kr is similar for both series, their values deduced in terms of D values for (Fe + Ni) samples are approximately six times larger than that deduced from EB values, which is not clear for us at present. Interestingly, the behavior of Kr is typically similar to JK behavior, i.e. (Kr \(\propto\) JK, Kr \(\propto\) 1/EB). This finding suggests that the lower values of Kr provide higher values of both α and EB, while the vice is versa for the higher Kr, in consistent with the reported elsewhere [57, 58].
The J-E relation of ZnO is given by [51, 59];
where C is constant and α is obtained from the slope of the I-V plot on a log–log scale for each sample [60]. The behavior of α against (Fe + M) and at different values of Ts shown in Fig. 6 is similar to the behavior of EB, while the vice is versa with Kr and JK behaviors, i.e. (α \(\propto\) EB, α \(\propto\) 1/Kr, α \(\propto\) 1/JK). These results indicated that the increase in Ts generally decreased the non-ohmic behaviors of the samples and moves the nonlinear parameters to lower values. Similar behavior is obtained for (Fe + Ni) samples at constant Ts, while the vice is versa for the behaviors of Fe and (Fe + Cu) samples. This is of course controlled by the height of potential barrier formed between ZnO grains which will be presented in the next paragraph. [56, 61,62,63,64]. For more understanding about the impact of co-doping on the values α as compared to individual dopants, the reported values of α for ZnO doped by Fe, Cu and Ni are enclosed in the same figure. Excluding the Fe = 0.30 sample, it is noted that the values of α for the co-doped samples are generally higher than that of dopants samples, which in general highlight the present investigation.
The electrical conductivity σ1 in the first ohmic region for the samples is calculated by using ohmic relation (σ1 = ∆J/∆E) and listed in Table 1. Although σ1 is obviously improved by increasing Ts for all samples, it is slightly decreased for Fe and (Fe + Cu) samples, but it is significantly increased for (Fe + Ni) samples. The density of carriers n is also calculated by; (σ1 = neμ), and 100 cm2 V−1 s −1 value was taken for the carrier mobility μ at 300 K, for simplicity [23, 65]. It is evident from the behavior of n shown in Fig. 7a that n has the same behavior of Kr deduced from D values rather than EB values. It is slightly decreased for Fe and (Fe + Cu) samples, while it is enhanced for (Fe + Ni) samples. This behavior is consistent with the reported data which indicated that Ni has increased the width of localized states of ZnO improved by Ni [55].
On the other hand, the electrical conductivity of the upturn region σL is calculated in terms of α and σ1 as follows [19];
where E1 and E2 are the applied fields around the boundaries of nonlinear region. It is observed from Fig. 7b that σL behaviors as follows; (σL \(\propto\) 1/EB, σL \(\propto\) α, σL \(\propto\) Kr). This result indicated that σL does not follow σ1 and it has a strange behavior (σL \(\propto\) 1/EB), but it is good evidence for the barrier formation such that (σL \(\propto\) α). For more clarification, the reduced conductivity (σR = σL/σ1) is plotted against co-doping content and shown in Fig. 7c. It is clear that the increase/decrease in the values of σR as a result of nonlinearity for the samples is similar to EB and α behavior. Our strong point is why the gain of conductivity is higher for Fe and (Fe + Cu) samples, as compared to (Fe + Ni) samples. Regardless of the number of electrons, we assumed that the mobility of electrons (σ = neμ) will be increased by increasing the applied field until the gain kinetic energy of electron is so enough to overcome the barrier. Consequently, the gain of conductivity across the nonlinear region for Fe and (Fe + Cu) samples could be evaluated as compared to (Fe + Ni) samples.
The J-E behavior of ZnO varistor in the ohmic region is given by [60, 66];
where A = (4ρemKB2/h3) is the Richardson’s constant, φB is the interface barrier height, ρ is the bulk density and β is a constant. The density of the samples ρexp is measured by using the Archimedes principle and listed in Table 1 for all samples. By measuring the J and keeping T constant for two different roots of E, φB could be obtained. Figure 8 shows the variation in φB against doping content at different values of Ts. It is found that the behavior of φB is similar to both α, EB and σL, and consequently supporting the strong formation of potential barriers in pure, Fe and (Fe + Cu) samples as compared to (Fe + Ni) samples.
Based on the above results, it is believed that Fe or (Fe + Cu) or (Fe + Ni) is well dissolved into the crystalline lattice of ZnO. During sintering at high temperature, the clusters of Fe or (Fe + Cu) samples behave deep donors, and therefore the density of the intrinsic donors will be reduced [61, 67, 68]. Furthermore, the higher valence state of the co-doping (Fe3+/Cu2+/3) compared to Zn2+ led to more deep acceptor levels which trap the electrons from the conduction band and then widening the energy gap. Moreover, it has been expected that (Fe + Cu) clusters may also form individual traces of oxides arising from the band structure and act as prime nonradiative recombination centers (native defects), and consequently a strong potential barrier could be formed [55, 57, 69, 70]. Consequently, σ1 and σL of the Fe/ (Fe + Cu) samples are slightly lower than that of the ZnO, while EB and α are higher. Therefore, a strong participation between Cu and Fe clusters may make this process more effective and also support the potential barrier of ZnO to be more resistive as obtained.
On the other hand, the (Fe + Ni) samples should have the same behavior of Fe samples due to divalent of Ni2+, which could not be obtained. As indicated above, the individual doping of Ni2+ in place of Zn2+ has completely deformed ZnO potential barrier as a result of highly localized cationic semicore d-states rich in Ni [71,72,73]. However, the addition of Ni beside Fe has not completely deformed the barrier even by 0.20 of Ni. Therefore, we believed that the participation of clusters for (Fe + Ni) samples also behaves as extrinsic donors, as well as Ni, but they are not sufficient for ZnO barrier deformation, as well as Ni. Consequently, the electrical conductivity is increased, while EB and α are decreased. It is approved that Ni helps for the formation of oxygen vacancies for (Fe + Ni) samples, and, therefore, the charge carrier density and also the ability of electron migration are significantly enhanced [74]. Meanwhile, the potential barrier will be decreased as a result of energy levels formed below the conducting band and consequently, the performance of ZnO conductivity is significantly improved [75]. Finally, the magnetic moments of Fe (3.36 µB) and Ni (3.2 µB), but Cu is nearly nonmagnetic. Nevertheless, the (Fe + Cu) samples show high nonlinear parameters as compared to (Fe + Ni) samples. Therefore, the addition of Cu beside Fe supports the barrier formation of ZnO as compared to Fe or Cu doping. In contrast, the addition of Ni beside Fe delays the barrier deformation of ZnO. Based on the above results, one can believe that the RTFM is not the unique reason responsible for the barrier deformation and some other reasons such as oxygen vacancies, valance state, recombination of electrons and holes may be responsible for the present behavior. To support our idea, the nonlinear parameters of the co-doped samples are higher than that of the individual dopants.
4 Conclusion
Structural morphology and nonlinear behavior of pure and co-doped Zn0.90-xFe0.1MxO with various M and x and at different Ts are well investigated. We have shown that both Fe, (Fe + Cu), (Fe + Ni) and also Ts did not deform the wurtzite structure of ZnO and also no secondary phases at the boundaries of grains could be formed. As compared to ZnO, the average grain size D is decreased for Fe and (Fe + Cu) samples, while it is increased for (Fe + Ni) samples. Although the nonlinear coefficient α and breakdown field EB of ZnO are improved by 0.1 of Fe, they are shifted to higher/lower values when Zn is replaced by (Fe + Cu)/(Fe + Ni) co-doping. Interestingly, values of α and EB for pure ZnO could be increased by (0.1Fe + 0.2Cu) from 47.02 and 2115.38 V/cm to 84.10 and 5012 V/cm, respectively, while they are decreased by (0.1Fe + 0.2Ni) to 11.85, 172.42 V/cm. Similar behavior is obtained for the nonlinear conductivity σL and the height of potential barrier φB, while the vice is versa for the behavior of leakage current Jk and residual voltage Kr. It is approved that the doping of (Fe + Cu) to ZnO supports the formation of potential barrier of ZnO as compared to (Fe + Ni) dopants, which highlight the present investigation.
References
H.M. Zeyada, M.M. EL-Nahass, I.S. El-Ashmawi, A.A. Habashi, J. Non-Cryst. Solids 358, 625–636 (2012)
N. Kumari, S. Naqvi, R. Kumar, J. Mater. Sci. 53, 4046–4055 (2018)
P.O.A.D. Nursaadah Ahmad, S.Z.N. Demon, M.Z. Yahya, N. Bidin, Int. J. Curr. Sci. Eng. Technol. 20, 1262–268 (2018)
M.H. Shahrokh Abadi, A. Delbari, Z. Fakoor, J. Baedi, J. Ceram, Sci. Technol. 6(1), 41 (2015)
B.L. Zhu, X.H. Sun, X.Z. Zhao, F.H. Su, G.H. Li, X.G. Wu, Vacuum 82, 495 (2008)
F. Obe, Y. Sato, T. Yamamoto, Y. Ikuhara, T. Sakuma, J. Am. Ceram. Soc. 86(9), 1 (2003)
Z. Zhou, K. Kato, T. Komaki, M. Yoshino, H. Yukawa, M. Morinaga, K. Morita, J. Eur. Ceram. Soc. 24, 139 (2004)
W.R.W. Abdullah, A. Zakaria, M.S.M. Ghazali, Int. J. Mol. Sci. 13(4), 5278–5289 (2012)
S. Anas, R.V. Mangalaraja, M. Poothayal, S.K. Shukla, S. Ananthakumar, Acta Mater. 55(17), 5792–5801 (2007)
M. Peiteado, J.F. Fernandez, A.C. Caballero, J. Eur. Ceram. Soc. 25(12), 2999–3003 (2005)
M. Peiteado, J.F. Fernandez, A.C. Caballero, J. Eur. Ceram. Soc. 27(13–15), 3867–3872 (2007)
F.K. Shan, Z.F. Liu, G.X. Liu, W.J. Lee, G.H. Lee, I.S. Kim, J. Electroceram. 13, 195 (2004)
A.B. Glot, J. Mater. Sci. Mater electron 17, 755 (2006)
B Ul. Haqa, A. Afaqa, R. Ahmedb, S. Naseema, Chin. Phys. B 21(9), 097101 (2012)
Z. Long, L. Peng-Fei, Y. Zhong-Yuan, M. Shi-Jia, D. Lu, L. Jian-Tao, Chin. Phys. B 21(9), 097103 (2012)
W.H. Pan, S.T. Kuo, W.H. Tuan, H.R. Chen, Int. J. Appl. Ceram. Tech. 7, E80 (2010)
W.R.W. Abdullah, A. Zakaria, M.S.M. Ghazali, Int. J. Mol. Sci. 13(4), 5278 (2012)
V.R. Shinde, T.P. Gujar, C.D. Lokhanade, R.S. Mane, S.H. Han, Mater. Chem. Phys. 96, 326 (2006)
Q. Shen, T. Toyoda, Jpn. J. Appl. Phys. 39, 3146 (2000)
S. Abdalla, K. Easawi, T.A. El-Brolossy, G.M. Yossef, S. Negm, H. Talaat, Rev. Sci. Instrum. 74(1), 848 (2003)
Q. Shen, T. Toyoda, Jpn. J. Appl. Phys. 39, 909 (2000)
T.A. El-Brolossy, S. Abdalla, O.E. Hassanein, S. Negm, H. Talaat, J. Phys IV France 125, 685 (2005)
J. Han, A.M.R. Senos, P.Q. Mantas, Mater. Chem. Phys. 75, 117 (2002)
A. Dutta, N. Gayathri, R. Ranganathan, Phys. Rev. B 68, 054432 (2003)
D. Ionescu, G. Venkataiah, Phys. B (375–377), 1040 (2005)
G. Pei, C. Xia, S. Cao, J. Zhang, F. Wu, J. Xu, J. Magn. Mater. 302(2), 340 (2006)
S.J. Kang, Y.H. Joung, H.H. Shin, Y.S. Yoon, J. Mater. Sci.: Mater. Electron. 19, 1073 (2008)
H.S. Kang, J.S. Kang, S.S. Pang, E.S. Shim, S.Y. Lee, Mater. Sci. Eng. B 102, 313 (2003)
A. Sedky, Brazilian J physics 44(4), 305 (2014)
Ü. Özgür, A. Ya, I. Alivov, C. Liu, A. Teke, M.A. Reshchikov, S. Doğan, C.V. Avrutin, S.J. Cho, A. Morkoçd, J. Appl. Phys. 98, 041301 (2005)
A. Sedky, S.B. Mohamed, Mater. Sci. Pol. 32(1), 16 (2014)
S. Aksoy, Y. Caglar, S. Ilican, M. Caglar, Adv. Control Chem. Eng. Civ. Eng. Mech. Eng. 227 (2010)
A. Sedky, M. Abu-Abdeen, A.A. Almulhem, Phys. B 388, 266 (2007)
A. Sedky, E. El-Suheel, Chin. Phys. B 21(11), 116103 (2012)
J.P. Joshi, R. Gupta, A.K. Sood, S.V. Bhat, A.R. Raju, C.N.R. Rao, Phys. Rev. B 65, 024410 (2001)
S.S. Dhar, O. Brandt, M. Ramsteiner, V.F. Sapega, K.H. Ploog, Phys. Rev. Lett. 94, 037305 (2005)
M. Mustaqima, C. Liu, Turk. J. Phys. 38, 429–441 (2014)
I. Djerdj, Z. Jaglici`c, D. Arcon, M. Niederberger, Nanoscale 2, 1096–1104 (2010)
Z. Yang, Appl. Phys. A 112, 241–254 (2013)
C. Liu, F. Yun, H. Morkoç, J. Mater. Sci. Mater. Electron. 16, 555–597 (2005)
J. Zhang, K. Tse, M. Wong, Y. Zhang, Zhu J Front. Phys. 11, 117405 (2016)
H. Katayama-Yoshida, T. Nishimatsu, T. Yamamoto, N. Orita, J. Phys. Condens. Matter 13, 8901–8914 (2001)
J. Hite, G.T. Thaler, R. Khanna, C.R. Abernathy, S.J. Pearton, J.H. Park, A.J. Steckl, J.M. Zavada, Appl. Phys. Lett. 89, 132119 (2006)
Y.K. Zhou, S.W. Choi, S. Kimura, S. Emura, S. Hasegawa, H. J. Asahi Supercond. Nov. Magn. 20, 429 (2007)
M. Ram, N.S. Negi, Phys. B Conden. Matter 481, 185 (2016)
M.S. Park, B.I. Min, Phys. Rev. B 68, 224436 (2003)
A. Sedky, N. Afify, Under Rev. J. Phys. Chem. Solids (2021)
A. Samanta, M.N. Goswami, P.K. Mahapatra, J. Alloys Comp. 730, 399 (2018)
A. Sedky, H. Mahfoz Kotb, Curr. Appl. Phys. 13, 2117 (2013)
A. Sedky, E. El-Suheel, Chin Phys B 21(11), 116103 (2012)
A. Sedky, E. El-Suheel, Phys Res Int 2010, 1 (2010)
U. Seetawan, S. Jugsujinda, T. Seetawan, A. Ratchasin, C. Euvananont, C. Junin, C. Thanachayanont, P. Chainaronk, Mater. Sci. Appl. 2, 1302 (2011)
E. Muchuweni, T.S. Sathiaraj, H. Nyakotyo, Heliyon 3(00285), 1 (2017)
A. Sedky, Adv Mater. Sci. Eng. 2(1), 1 (2018)
E.R. Shaaban, M.M. Soraya, M. Shapaan, H. Shokry Hassan, M.M. Samar, J. Alloy. Compd. 693, 1052 (2017)
J.W. Fergus, J. Mater. Sci. 38, 4259 (2003)
H.M. Ali, A.M. Abdel Hakeem, Phys. Status Solidi A 207(1), 132 (2010)
A. Sedky, T.A. El-Trabolosy, S.B. Mohamed, J. Phys. Chem. Solids 73, 505 (2012)
X.S. Wang, Z.C. Wu, J.F. Webb, Z.G. Liu, Appl. Phys. A 77, 561 (2003)
W.R.W. Abdullah, A. Zakaria, M.S.M. Ghazali, Int. J. Mol. Sci. 13, 5278 (2012)
S. Li, F. Xie, F. Liu, J. Li, M.A. Alim, Mater. Lett. 59, 302 (2005)
A. Sedky, Int. J. Photon. Opt. Technol. 3(3), 1 (2017)
Y.G. Wang, S.P. Lau, H.W. Lee, S.F. Yu, S.K. Tay, X.Z. Zang, H.H. Hing, J. Appl. Phys. 94, 354 (2003)
Xuhai Li, Xiuxia Cao, Xu. Liang, L. Liu, Y. Wang, C. Meng, Z. Wang, J. Alloys Comp. 675, 90 (2016)
A. Sedky, S.A. Amin, M. Mohamed, Appl. Phys. A 125, 308 (2019)
S.R. Dhage, V. Choube, V. Ravi, Mater. Sci. Eng. B 110, 168 (2004)
G. Patwari, P. Kumar Kalita, R. Singha, Mater. Sci.-Poland 34(1), 69–78 (2016). https://doi.org/10.1515/msp-2016-0030
S.S. Abdullahi, Y. Köseoğlu, S. Güner, S. Kazan, B. Kocaman, C.E. Ndikilar, Superlatt. Microstruct. 83, 342–352 (2015)
H. Cao, P. Lu, N. Cai, X. Zhang, Z. Yu, T. Gao, S. Wang, J. Magn. Magn. Mater. 352, 66 (2014)
J. Han, P.Q. Mantas, A.M.R. Senos, J. Eur. Ceram. Soc. 21, 1883 (2001)
H.M. Ali, H.A. Mohamed, S.H. Mohamed, Eur. Phys. J. Appl. Phys. 31, 87 (2005)
A. Sedky, E. El-Suheel, Phys. Res. Int. 1, 1 (2010)
D. Sharma, R. Jha, Transition metal (Co, Mn) co-doped ZnO nanoparticles. J. Alloys Comp. 698, 532–538 (2017)
R.A. Ismail, A.K. Ali, M.M. Ismail, K.I. Hassoon, Appl Nanosci 1, 45–49 (2011)
B. Yuyu, Z. Chen, J. Power Sour. 272, 647 (2014)
Acknowledgements
Authors acknowledge the Deanship of Scientific Research, King Faisal University for their support under grant number [17122004].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Naim, A.F., Afify, N., Sedky, A. et al. Structural morphology and nonlinear behavior of pure and co-doped Zn1-x-yFexMyO varistors with (M = Cu, Ni). Appl. Phys. A 127, 486 (2021). https://doi.org/10.1007/s00339-021-04560-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04560-3