Abstract
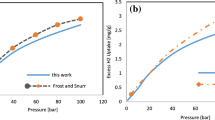
Isosteric heat of adsorption is an important parameter required to describe the thermal performance of adsorptive storage systems. It is most frequently calculated from adsorption isotherms measured over wide ranges of pressure and temperature, using the so-called adsorption isosteric method. Direct quantitative estimation of isosteric heats on the other hand is possible using the coupled calorimetric–volumetric method, which involves simultaneous measurement of heat and adsorption. In this work, we compare the isosteric heats of hydrogen adsorption on microporous materials measured by both methods. Furthermore, the experimental data are compared with the isosteric heats obtained using the modified Dubinin–Astakhov, Tóth, and Unilan adsorption analytical models to establish the reliability and limitations of simpler methods and assumptions. To this end, we measure the hydrogen isosteric heats on five prototypical metal–organic frameworks: MOF-5, Cu-BTC, Fe-BTC, MIL-53, and MOF-177 using both experimental methods. For all MOFs, we find a very good agreement between the isosteric heats measured using the calorimetric and isosteric methods throughout the range of loading studied. Models’ prediction on the other hand deviates from both experiments depending on the MOF studied and the range of loading. Under low-loadings of less than 5 mol kg−1, the isosteric heat of hydrogen adsorption decreases in the order Cu-BTC > MIL-53 > MOF-5 > Fe-BTC > MOF-177. The order of isosteric heats is coherent with the strength of hydrogen interaction revealed from previous thermal desorption spectroscopy measurements.



Similar content being viewed by others
References
J.K.N. Newhouse, in Proceedings of Annual Merit Review Meetings of US DOE (2014), http://www.hydrogen.energy.gov/pdfs/review14/st047_newhouse_2014_o.pdf
Y.-S. Bae, R.Q. Snurr, Microporous Mesoporous Mater. 132, 300 (2010)
B. Hardy, C. Corgnale, R. Chahine, M.-A. Richard, S. Garrison, D. Tamburello, D. Cossement, D. Anton, Int. J. Hydrogen Energy 37, 5691 (2012)
S. Ubaid, R. Zacharia, J. Xiao, R. Chahine, P. Bénard, P. Tessier, Int. J. Hydrogen Energy 40, 9314 (2015)
J. Bear, Y. Bachmat, Introduction to Modeling of Transport Phenomena in Porous Media (Kluwer Academic Publishers, The Netherlands, 1991)
M.-A. Richard, D. Cossement, P.-A. Chandonia, R. Chahine, D. Mori, K. Hirose, AIChE J. 55, 2985 (2009)
E. Dundar, R. Zacharia, R. Chahine, P. Benard, Fluid Phase Equilibr 363, 74 (2014)
J. Xiao, L. Tong, C. Deng, P. Bénard, R. Chahine, Int. J. Hydrogen Energy 35, 8106 (2010)
L.J. Murray, M. Dinca, J.R. Long, Chem. Soc. Rev. 38, 1294 (2009)
J. Purewal, D. Liu, A. Sudik, M. Veenstra, J. Yang, S. Maurer, U. Müller, D.J. Siegel, J. Phys. Chem. C 116, 20199 (2012)
A. Dailly, J.J. Vajo, C.C. Ahn, J. Phys. Chem. B 2006, 110 (1099)
B. Panella, M. Hirscher, H. Pütter, U. Müller, Adv. Funct. Mater. 16, 520 (2006)
B. Schmitz, U. Müller, N. Trukhan, M. Schubert, G. Férey, M. Hirscher, ChemPhysChem 9, 2181 (2008)
W. Zhou, H. Wu, M.R. Hartman, T. Yildirim, J. Phys. Chem. C 111, 16131 (2007)
S.S. Kaye, A. Dailly, O.M. Yaghi, J.R. Long, J. Am. Chem. Soc. 129, 14176 (2007)
M. Schlichtenmayer, B. Streppel, M. Hirscher, Int. J. Hydrogen Energy 36, 586 (2011)
S. Ozawa, S. Kusumi, Y. Ogino, J. Colloid Interface Sci. 56, 83 (1976)
A. Züttel, P. Sudan, P. Mauron, P. Wenger, Appl. Phys. A 78, 941 (2004)
N.P. Stadie, Dissertation (Ph.D.), California Institute of Technology (2013)
J. Purewal, Dissertation (Ph.D.), California Institute of Technology (2010)
W. Zimmermann, J.U. Keller, Thermochim. Acta 405, 31 (2003)
E.W. Lemmon, M.L. Huber, M.O. McLinden, in NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 9.1, National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg, 2013 (National Institute of Standards and Technology, 2013)
P.B. Whittaker, X. Wang, K. Regenauer-Lieb, H.T. Chua, Phys. Chem. Chem. Phys. 15, 473 (2013)
R. Zacharia, D. Cossement, L. Lafi, R. Chahine, J. Mater. Chem. 20, 2145 (2010)
E. Poirier, R. Chahine, P. Bénard, L. Lafi, G. Dorval-Douville, P.A. Chandonia, Langmuir 22, 8784 (2006)
G. Dorval-Douville, Master Thesis, Université du Québec a trois rivieres (2006)
A. Mouahid, D. Bessieres, F. Plantier, G. Pijaudier-Cabot, J. Therm. Anal. Calorim. 2012, 109 (1077)
L.E. Vilchiz-Bravo, A. Pacheco-Vega, B.E. Handy, Meas. Sci. Technol. 21, 115103 (2010)
L.E. Vilchiz, A. Pacheco-Vega, B.E. Handy, Thermochim. Acta 439, 110 (2005)
J.A. Dunne, M. Rao, S. Sircar, R.J. Gorte, A.L. Myers, Langmuir 12, 5896 (1996)
K.Y. Foo, B.H. Hameed, Chem. Eng. J. 156, 2 (2010)
F.o. Rouquerol, J. Rouquerol, K.S.W. Sing, Adsorption by Powders and Porous Solids: Principles, Methodology, and Applications (Academic Press, San Diego, 1999)
M. Schlichtenmayer, M. Hirscher, J. Mater. Chem. 22, 10134 (2012)
S. Bourrelly, P.L. Llewellyn, C. Serre, F. Millange, T. Loiseau, G. Férey, J. Am. Chem. Soc. 127, 13519 (2005)
S. Maurice, Ph.D. Thesis, Max-Planck-Institut für Intelligente Systeme, Stuttgart (2012)
B. Panella, K. Hones, U. Muller, N. Trukhan, M. Schubert, H. Putter, M. Hirscher, Angew. Chem. Int. Edit. 47, 2138 (2008)
I. Krkljus, M. Hirscher, Microporous Mesoporous Mater. 142, 725 (2011)
Acknowledgments
The Canadian contribution is based on research financially supported by NSERC and Air Liquide. The German contribution was partially funded by the German Research Foundation (SPP 1362), the European Commission DG Research (SES6-2006-518271/NESSHY) and the European Hy-Co program, financed by the German Federal Ministry of Economics and Technology (BMWi).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
339_2015_9484_MOESM1_ESM.docx
Details of adsorption calorimetry, isosteric method, volume calibration and adsorption isotherms are available (DOCX 1249 kb)
Rights and permissions
About this article
Cite this article
Kloutse, A.F., Zacharia, R., Cossement, D. et al. Isosteric heat of hydrogen adsorption on MOFs: comparison between adsorption calorimetry, sorption isosteric method, and analytical models. Appl. Phys. A 121, 1417–1424 (2015). https://doi.org/10.1007/s00339-015-9484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9484-6