Abstract
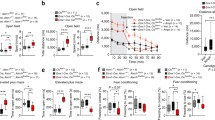
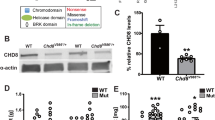
Sequences encoding DUF1220 protein domains show the most extreme human lineage-specific copy number increase of any coding region in the genome and have been linked to human brain evolution. In addition, DUF1220 copy number (dosage) has been implicated in influencing brain size within the human species, both in normal populations and in individuals associated with brain size pathologies (1q21-associated microcephaly and macrocephaly). More recently, increasing dosage of a subtype of DUF1220 has been linked with increasing severity of the primary symptoms of autism. Despite these intriguing associations, a function for these domains has not been described. As a first step in addressing this question, we have developed the first transgenic model of DUF1220 function by removing the single DUF1220 domain (the ancestral form) encoded in the mouse genome. In a hypothesis generating exercise, these mice were evaluated by 197 different phenotype measurements. While resulting DUF1220-minus (KO) mice show no obvious anatomical peculiarities, they exhibit a significantly reduced fecundity (χ 2 = 19.1, df = 2, p = 7.0 × 10−5). Further extensive phenotypic analyses suggest hyperactivity (p < 0.05) of DUF1220 mice and changes in gene expression levels of brain associated with distinct neurological functions and disease. Other changes that met statistical significance include an increase in plasma glucose concentration (as measured by area under the curve, AUC 0–30 and AUC 30–120) in male mutants, fasting glucose levels, reduce sodium levels in male mutants, increased levels of the liver functional indicator ALAT/GPT in males, levels of alkaline phosphatase (also an indicator of liver function), mean R and SR amplitude by electrocardiography, elevated IgG3 levels, a reduced ratio of CD4:CD8 cells, and a reduced frequency of T cells; though it should be noted that many of these differences are quite small and require further examination. The linking of DUF1220 loss to a hyperactive phenotype is consistent with separate findings in which DUF1220 over expression results in a down-regulation of mitochondrial function, and potentially suggests a role in developmental metabolism. Finally, the substantially reduced fecundity we observe associated with KO mice argues that the ancestral DUF1220 domain provides an important biological functionthat is critical to survivability and reproductive success.




Similar content being viewed by others
References
Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, Costa SM, Baralle D, Raponi M, Karbani G, Rashid Y, Jafri H, Bennett C, Corry P, Walsh CA, Woods CG (2005) A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet 37(4):353–355
Conforti L, Buckmaster EA, Tarlton A, Brown MC, Lyon MF, Perry VH, Coleman MP (1999) The major brain isoform of kif1b lacks the putative mitochondria-binding domain. Mamm Genome 10:617–622
Davis JM, Searles VB, Anderson N, Keeney J, Dumas L, Sikela JM (2014) DUF1220 dosage is linearly associated with increasing severity of the three primary symptoms of autism. PLoS Genetics 10(3):e1004241
Dumas LJ, O’Bleness MS, Davis JM, Dickens CM, Anderson N, Keeney JG, Jackson J, Sikela M, Raznahan A, Giedd J, Rapoport J, Nagamani SS, Erez A, Brunetti-Pierri N, Sugalski R, Lupski JR, Fingerlin T, Cheung SW, Sikela JM (2012) DUF1220-domain copy number implicated in human brain-size pathology and evolution. Am J Hum Genet 91(3):444–454
Dumont M, Stack C, Elipenahli C, Jainuddin S, Launay N, Gerges M, Starkova N, Starkov AA, Calingasan NY, Tampellini D, Pujol A, Beal MF (2014) PGC-1alpha overexpression exacerbates beta-amyloid and tau deposition in a transgenic mouse model of Alzheimer’s disease. FASEB J 28:1745–1755
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210
Fuchs H1, Gailus-Durner V, Adler T, Aguilar-Pimentel JA, Becker L, Calzada-Wack J, Da Silva-Buttkus P, Neff F, Götz A, Hans W, Hölter SM, Horsch M, Kastenmüller G, Kemter E, Lengger C, Maier H, Matloka M, Möller G, Naton B, Prehn C, Puk O, Rácz I, Rathkolb B, Römisch-Margl W, Rozman J, Wang-Sattler R, Schrewe A, Stöger C, Tost M, Adamski J, Aigner B, Beckers J, Behrendt H, Busch DH, Esposito I, Graw J, Illig T, Ivandic B, Klingenspor M, Klopstock T, Kremmer E, Mempel M, Neschen S, Ollert M, Schulz H, Suhre K, Wolf E, Wurst W, Zimmer A, Hrabě de Angelis M (2011) Mouse phenotyping. Methods 53(2):120–35
Gailus-Durner V1, Fuchs H, Adler T, Aguilar Pimentel A, Becker L, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Ferwagner B, Hans W, Hölter SM, Hölzlwimmer G, Horsch M, Javaheri A, Kallnik M, Kling E, Lengger C, Mörth C, Mossbrugger I, Naton B, Prehn C, Puk O, Rathkolb B, Rozman J, Schrewe A, Thiele F, Adamski J, Aigner B, Behrendt H, Busch DH, Favor J, Graw J, Heldmaier G, Ivandic B, Katus H, Klingenspor M, Klopstock T, Kremmer E, Ollert M, Quintanilla-Martinez L, Schulz H, Wolf E, Wurst W, de Angelis MH (2009) Systemic first-line phenotyping. Methods Mol Biol 530:463–509
Gailus-Durner V, Fuchs H, Becker L, Bolle I, Brielmeier M, Calzada-Wack J, Elvert R, Ehrhardt N, Dalke C, Franz TJ, Grundner-Culemann E, Hammelbacher S, Hölter SM, Hölzlwimmer G, Horsch M, Javaheri A, Kalaydjiev SV, Klempt M, Kling E, Kunder S, Lengger C, Lisse T, Mijalski T, Naton B, Pedersen V, Prehn C, Przemeck G, Racz I, Reinhard C, Reitmeir P, Schneider I, Schrewe A, Steinkamp R, Zybill C, Adamski J, Beckers J, Behrendt H, Favor J, Graw J, Heldmaier G, Höfler H, Ivandic B, Katus H, Kirchhof P, Klingenspor M, Klopstock T, Lengeling A, Müller W, Ohl F, Ollert M, Quintanilla-Martinez L, Schmidt J, Schulz H, Wolf E, Wurst W, Zimmer A, Busch DH, de Angelis MH (2005) Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat Methods 2(6):403–404
Granchi C, Bertini S, Macchia M, Minutolo F (2010) Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem 17(7):672–697
Hellier JL, Arevalo NL, Blatner MJ, Dang AK, Clevenger AC, Adams CE, Restrepo D (2010) Olfactory discrimination varies in mice with different levels of α7-nicotinic acetylcholine receptor expression. Brain Res 1358:140–150
Horsch M, Schadler S, Gailus-Durner V, Fuchs H, Meyer H, de Angelis MH, Beckers J (2008) Systematic gene expression profiling of mouse model series reveals coexpressed genes. Proteomics 8:1248–1256
Keeney JG, Davis JM, Siegenthaler J, Post MD, Nielsen BS, Hopkins WD, Sikela JM (2014) DUF1220 protein domains drive proliferation in human neural stem cells and are associated with increased cortical volume in anthropoid primates. Brain Struct Funct. doi:10.1007/s00429-014-0814-9
Kugler JE, Horsch M, Huang D, Furusawa T, Rochman M, Garrett L, Becker L, Bohla A, Holter SM, Prehn C, Rathkolb B, Racz I, Aguilar-Pimentel JA, Adler T, Adamski J, Beckers J, Busch DH, Eickelberg O, Klopstock T, Ollert M, Stoger T, Wolf E, Wurst W, Yildirim AO, Zimmer A, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Garfinkel B, Orly J, Ovcharenko I, Bustin M (2013) High mobility group N proteins modulate the fidelity of the cellular transcriptional profile in a tissue- and variant-specific manner. J Biol Chem 288:16690–16703
Llorens-Martin M, Lopez-Domenech G, Soriano E, Avila J (2011) GSK3beta is involved in the relief of mitochondria pausing in a Tau-dependent manner. PLoS One 6:e27686
O’Bleness MS, Dickens CM, Dumas LJ, Kehrer-Sawatzki H, Wyckoff GJ, Sikela JM (2012) Evolutionary history and genome organization of DUF1220 protein domains. G3 (Bethesda) 2(9):977–986
Popesco MC, Maclaren EJ, Hopkins J, Dumas L, Cox M, Meltesen L, McGavran L, Wyckoff GJ, Sikela JM (2006) Human lineage-specific amplification, selection, and neuronal expression of DUF1220 domains. Science 313(5791):1304–1307
Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL (2008) Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci 28(3):625–632
Slotnick B, Restrepo D (2005) Olfactometry with mice. Current Protocols in Neuroscience, Chapter 8, Unit 8.20
Uys GM, Ramburan A, Loos B, Kinnear CJ, Korkie LJ, Mouton J, Riedemann J, Moolman-Smook JC (2011) Myomegalin is a novel A-kinase anchoring protein involved in the phosphorylation of cardiac myosin binding protein C. BMC Cell Biol 12:18
Vandepoele K, Van Roy N, Staes K, Speleman F, van Roy F (2005) A novel gene family NBPF: intricate structure generated by gene duplications during primate evolution. Mol Biol Evol 22(11):2265–2274
Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M (2001) Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem 276(14):11189–11198
Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Müller MJ (2010) Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92(6):1369–1377
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Xu W, Zhu H, Gu M, Luo Q, Ding J, Yao Y, Chen F, Wang Z (2013) DHTKD1 is essential for mitochondrial biogenesis and function maintenance. FEBS Lett 587:3587–3592
Acknowledgments
This works was supported in part by Grants 5R01 MH081203-05, 3R01 MH08120302S1, 5R21-R33 MH089917-03 to JMS, and by a Graduate Student Fellowship from the Coleman Institute for Cognitive Disabilities. This work was also supported by the the Deutsche Forschungsgemeinschaft (German Research Foundation) within the framework of the Munich Cluster for Systems Neurology (EXC 1010 SyNergy), funds from the German Federal Ministry of Education and Research (German Center for Vertigo and Balance Disorders, Grant 01 EO 0901; Infrafrontier, Grant 01KX1012), and the German Center for Diabetes Research (DZD).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
German Mouse Clinic Consortium: The members of the German Mouse Clinic Consortium are listed in the Appendix.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
German Mouse Clinic, Helmholtz Zentrum München, German Research Center for Environmental Health GmbH, Neuherberg, Germany
-
Thure Adler 1,2
-
Antonio Aguilar-Pimentel1,3
-
Oana Amarie1,4
-
Lore Becker 1,5
-
Johannes Beckers1,18,19
-
Raffi Bekeredjian6
-
Dirk H. Busch 2
-
Oliver Eickelberg 4
-
Lillian Garrett1,8
-
Jochen Graw 8
-
Wolfgang Hans1
-
Sabine M. Hölter 1,8
-
Marion Horsch1
-
Dirk Janik1,7
-
Martin Klingenspor9,10
-
Thomas Klopstock 5,16,20,21
-
Kristin Moreth1
-
Frauke Neff1,7
-
Markus Ollert3,11
-
Oliver Puk1,8
-
Ildikó Rácz1,12
-
Birgit Rathkolb 1,13,19
-
Jan Rozman 1,19
-
Tobias Stöger1,4
-
Eckhard Wolf 13
-
Wolfgang Wurst 8,14,15,16,17,21
-
Ali Önder Yildrim4
-
Andreas Zimmer12
-
Martin Hrabě de Angelis 1,18,19
-
Valérie Gailus-Durner 1
-
Helmut Fuchs 1
-
1.
German Mouse Clinic, Institute of Experimental Genetics, Helmholtz Zentrum München, German Research Center for Environmental Health GmbH, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
-
2.
Institute for Medical Microbiology, Immunology and Hygiene, Technical University of Munich, Trogerstrasse 9, 81675 Munich, Germany
-
3.
Department of Dermatology and Allergy, Biederstein, Klinikum rechts der Isar, Technische Universität München (TUM), Biedersteiner Str. 29, 80802 Munich,
-
4.
Comprehensive Pneumology Center, Institute of Lung Biology and Disease, Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH), Ingolstädter Landstraße 1, 85764 Neuherberg, Germany and Member of the German Center for Lung Research
-
5.
Department of Neurology, Friedrich-Baur-Institut, Ludwig-Maximilians-Universität München, Ziemssenstrasse 1a, 80336 Munich, Germany
-
6.
Department of Cardiology, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany
-
7.
Institute of Pathology, Helmholtz Zentrum München, German Research Center for Environmental Health GmbH, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
-
8.
Institute of Developmental Genetics, Helmholtz Zentrum München, German Research Center for Environmental Health GmbH, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
-
9.
Chair for Molecular Nutritional Medicine, Technische Universität München, Else Kröner-Fresenius Center for Nutritional Medicine, 85350 Freising, Germany
-
10.
ZIEL – Center for Nutrition and Food Sciences, Technische Universität München, 85350 Freising, Germany
-
11.
Clinical Research Group Molecular Allergology, Center of Allergy and Environment Munich (ZAUM), Technische Universität München (TUM), and Institute for Allergy Research, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg, Germany
-
12.
Institute of Molecular Psychiatry, University of Bonn, Sigmund-Freud-Strasse 25, 53127 Bonn, Germany
-
13.
Ludwig-Maximilians-Universität München, Gene Center, Institute of Molecular Animal Breeding and Biotechnology, Feodor-Lynen Strasse 25, 81377 Munich, Germany
-
14.
Chair of Developmental Genetics, Center of Life and Food Sciences Weihenstephan, Technische Universität München, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
-
15.
Max Planck Institute of Psychiatry, Kraepelinstr. 2-10, 80804 Munich, Germany
-
16.
Deutsches Institut für Neurodegenerative Erkrankungen (DZNE) Site Munich, Schillerstrasse 44, 80336 Munich, Germany
-
17.
Munich Cluster for Systems Neurology (SyNergy), Adolf-Butenandt-Institut, Ludwig-Maximilians-Universität München, Schillerstrasse 44, 80336 Munich, Germany
-
18.
Chair of Experimental Genetics, Center of Life and Food Sciences Weihenstephan, Technische Universität München, Ingolstaedter Landstrasse 1, 85764 Neuherberg, Germany
-
19.
Member of German Center for Diabetes Research (DZD), Ingolstaedter Landstraße 1, 85764 Neuherberg, Germany
-
20.
German Network for Mitochondrial Disorders (mitoNET)
-
21.
German Center for Vertigo and Balance Disorders, Munich, Germany
Rights and permissions
About this article
Cite this article
Keeney, J.G., O’Bleness, M.S., Anderson, N. et al. Generation of Mice Lacking DUF1220 Protein Domains: Effects on Fecundity and Hyperactivity. Mamm Genome 26, 33–42 (2015). https://doi.org/10.1007/s00335-014-9545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-014-9545-8