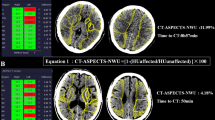
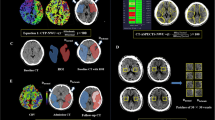
Abstract
Objective
To assess the role of net water uptake (NWU) in predicting outcomes in acute ischemic stroke (AIS) patients.
Methods
A systematic review and meta-analysis were performed, adhering to established guidelines. The search covered PubMed, Scopus, Web of Science, and Embase databases until July 1, 2023. Eligible studies reporting quantitative ischemic lesion NWU in admission CT scans of AIS patients, stratified based on outcomes, were included. Data analysis was performed using R software version 4.2.1.
Results
Incorporating 17 original studies with 2217 AIS patients, NWU was significantly higher in patients with poor outcomes compared to those with good outcomes (difference of medians: 5.06, 95% CI: 3.00–7.13, p < 0.001). Despite excluding one outlier study, considerable heterogeneity persisted among the included studies (I2 = 90.8%). The meta-regression and subgroup meta-analyses demonstrated significantly higher NWU in patients with poor functional outcome, as assessed by modified Rankin Scale (difference of medians: 3.83, 95% CI: 1.98–5.68, p < 0.001, I2 = 72.9%), malignant edema/infarct (difference of medians: 8.30, 95% CI: 4.01–12.58, p < 0.001, I2 = 95.6%), and intracranial hemorrhage (difference of medians: 5.43, 95% CI: 0.44–10.43, p = 0.03, I2 = 91.1%).
Conclusion
NWU on admission CT scans shows promise as a predictive marker for outcomes in AIS patients. Prospective, multicenter trials with standardized, automated NWU measurement are crucial for robustly predicting diverse clinical outcomes.
Clinical relevance statement
The potential of net water uptake as a biomarker for predicting outcomes in acute ischemic stroke patients holds significant promise. Further validation through additional research could lead to its integration into clinical practice, potentially improving the accuracy of clinical decision-making and allowing for the development of more precise patient care strategies.
Key Points
• Net water uptake, a CT-based biomarker, quantifies early brain edema after acute ischemic stroke.
• Net water uptake is significantly higher in poor outcome acute ischemic stroke patients.
• Net water uptake on CT scans holds promise in predicting diverse acute ischemic stroke outcomes.


Similar content being viewed by others
Abbreviations
- AIS:
-
Acute ischemic stroke
- ASPECTS:
-
Alberta stroke program early computed tomography score
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- CTP:
-
Perfusion CT
- ICH:
-
Intracranial hemorrhage
- MCE:
-
Malignant cerebral edema
- mRS:
-
Modified Rankin Scale
- NWU:
-
Net water uptake
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- ROC:
-
Receiver operating characteristic
References
Broocks G, Flottmann F, Scheibel A et al (2018) Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke 49:1906–1912
Broocks G, Flottmann F, Ernst M et al (2018) Computed tomography-based imaging of voxel-wise lesion water uptake in ischemic brain: relationship between density and direct volumetry. Invest Radiol 53:207–213
Zhang X, Huang P, Zhang R (2021) Evaluation and prediction of post-stroke cerebral edema based on neuroimaging. Front Neurol 12:763018
Sheth KN, Petersen NH, Cheung K et al (2018) Long-term outcomes in patients aged < / =70 years with intravenous glyburide from the phase II GAMES-RP study of large hemispheric infarction: an exploratory analysis. Stroke 49:1457–1463
Cook AM, Morgan Jones G, Hawryluk GWJ et al (2020) Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care 32:647–666
Gotoh O, Asano T, Koide T, Takakura K (1985) Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: the time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke 16:101–109
Walberer M, Blaes F, Stolz E et al (2007) Midline-shift corresponds to the amount of brain edema early after hemispheric stroke–an MRI study in rats. J Neurosurg Anesthesiol 19:105–110
Cheng X, Shi J, Wu H, Zhu W, Lu G (2022) Review of net water uptake in the management of acute ischemic stroke. Eur Radiol 32:5517–5524
Xu HB, Sun YF, Luo N et al (2021) Net water uptake calculated in standardized and blindly outlined regions of the middle cerebral artery territory predicts the development of malignant edema in patients with acute large hemispheric infarction. Front Neurol 12:645590
Minnerup J, Broocks G, Kalkoffen J et al (2016) Computed tomography-based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: a multicenter observational study. Ann Neurol 80:924–934
Nawabi J, Flottmann F, Kemmling A et al (2021) Elevated early lesion water uptake in acute stroke predicts poor outcome despite successful recanalization – when “tissue clock” and “time clock” are desynchronized. Int J Stroke 16:863–872
Nawabi J, Kniep H, Schön G et al (2019) Hemorrhage after endovascular recanalization in acute stroke: lesion extent, collaterals and degree of ischemic water uptake mediate tissue vulnerability. Front Neurol 10:569
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Wells GA, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 21 Oct 2023
McGrath S, Sohn H, Steele R, Benedetti A (2020) Meta-analysis of the difference of medians. Biom J 62:69–98
McGrath S, Zhao X, Ozturk O, Katzenschlager S, Steele R, Benedetti A (2023) metamedian: an R package for meta-analyzing studies reporting medians. arXiv Available via: https://arxiv.org/abs/2302.14243. Accessed 20 July 2023
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36:1–48
McGrath S, Katzenschlager S, Zimmer AJ, Seitel A, Steele R, Benedetti A (2023) Standard error estimation in meta-analysis of studies reporting medians. Stat Methods Med Res 32:373–388
McGrath S, Sohn H, Steele R, Benedetti A (2020) Meta-analysis of the difference of medians. Biom J 62:69–98
Vorasayan P, Bevers MB, Beslow LA et al (2019) Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke 50:3021–3027
Broocks G, Elsayed S, Kniep H et al (2021) Early prediction of malignant cerebellar edema in posterior circulation stroke using quantitative lesion water Uptake. Neurosurgery 88:531–537
Broocks G, Kemmling A, Teßarek S et al (2022) Quantitative lesion water uptake as stroke imaging biomarker: a tool for treatment selection in the extended time window? Stroke 53:201–209
Lu SS, Wu RR, Cao YZ et al (2022) ASPECTS-based net water uptake predicts poor reperfusion and poor clinical outcomes in patients with ischemic stroke. Eur Radiol 32:7026–7035
Han Q, Yang J, Gao X et al (2022) Early edema within the ischemic core is time-dependent and associated with functional outcomes of acute ischemic stroke patients. Front Neurol 13:861289
Broocks G, Meyer L, Elsayed S et al (2023) Association between net water uptake and functional outcome in patients with low ASPECTS brain lesions: Results from the I-LAST study. Neurology 100:e954–e963
Haupt W, Meyer L, Wagner M et al (2023) Assessment of irreversible tissue injury in extensive ischemic stroke-potential of quantitative cerebral perfusion. Transl Stroke Res 14:562–571
Fu B, Qi S, Tao L et al (2020) Image patch-based net water uptake and radiomics models predict malignant cerebral edema after ischemic stroke. Front Neurol 11:609747
Xia H, Sun H, He S et al (2021) Absent cortical venous filling is associated with aggravated brain edema in acute ischemic stroke. AJNR Am J Neuroradiol 42:1023–1029
Broocks G, Hanning U, Faizy TD et al (2020) Ischemic lesion growth in acute stroke: water uptake quantification distinguishes between edema and tissue infarct. J Cereb Blood Flow Metab 40:823–832
Broocks G, McDonough R, Meyer L et al (2021) Reversible ischemic lesion hypodensity in acute stroke CT following endovascular reperfusion. Neurology 97:e1075–e1084
Xu T, Yang J, Han Q et al (2022) Net water uptake, a neuroimaging marker of early brain edema, as a predictor of symptomatic intracranial hemorrhage after acute ischemic stroke. Front Neurol 13:903263
Shi J, Wu H, Dong Z et al (2022) Automated quantitative lesion water uptake in acute stroke is a predictor of malignant cerebral edema. Eur Radiol 32:2771–2780
Strbian D, Meretoja A, Putaala J, Kaste M, Tatlisumak T (2013) Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke 8:529–534
Dzialowski I, Klotz E, Goericke S et al (2007) Ischemic brain tissue water content: CT monitoring during middle cerebral artery occlusion and reperfusion in rats. Radiology 243:720–726
Kimberly WT, Dutra BG, Boers AMM et al (2018) Association of reperfusion with brain edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN Trial. JAMA Neurol 75:453–461
Simard JM, Kent TA, Chen M et al (2007) Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 6:258–268
Yaghi S, Willey JZ, Cucchiara B et al (2017) Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 48:e343–e361
Bernardo-Castro S, Sousa JA, Brás A et al (2020) Pathophysiology of blood-brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol 11:594672
Acknowledgements
We extend our appreciation to the Nested Knowledge developers, Karl Holub, Stephen Mead, Jeff Johnson, and Darian Lehmann-Plantenberg, for their contributions in enabling this study through the development of the AutoLit and Synthesis platforms for systematic review. We would also like to acknowledge the assistance provided by ChatGPT, an OpenAI language model based on the GPT-3.5 architecture, in language corrections during the manuscript editing process, which enhanced readability and language quality. However, the authors bear full responsibility for the content of this publication as they reviewed and edited the material after utilizing the tool.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is David F. Kallmes.
Conflict of interest
David F. Kallmes holds equity in Nested Knowledge, Superior Medical Editors, Conway Medical, Marblehead Medical, and Piraeus Medical. He receives grant support from MicroVention, Medtronic, Balt, and Insera Therapeutics. Additionally, he has served on the Data Safety Monitoring Board for Vesalio and has received royalties from Medtronic.
Ramanathan Kadirvel is contracted or acts as a consultant for the following companies: Cerenovus Inc, Medtronic, Endovascular Engineering, Frontior Bio, Sensome Inc, Endomimetics, Ancure LLC, Neurogami Medical, MIVI Biosciences, Monarch Biosciences, Stryker Inc, Conway Medical, Pireus Medical, and Bionau Labs. He holds research grants from the National Institutes of Health (NIH) for projects with grant numbers R01NS076491, R44NS107111, R43NS110114, and R21NS128199. He also holds a research grant from the National Science Foundation (NSF) with grant number 081215707. The other authors affirm that they have no competing interests to declare.
The remaining authors of this manuscript have disclosed no affiliations with companies whose products or services pertain to the article’s subject matter.
Statistics and biometry
Payam Jannatdoust and Parya Valizadeh made substantial contributions to data analysis and interpretation, while Sherief Ghozy, with notable statistical expertise, offered guidance on statistical aspects.
Informed consent
Written informed consent was not required for this study because it is a systematic review and meta-analysis study.
Ethical approval
Institutional Review Board approval was unnecessary, given the nature of this systematic review and meta-analysis study.
Study subjects or cohorts overlap
No study subjects or cohorts overlap have been previously reported.
Methodology
• Multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sherief Ghozy, Melika Amoukhteh, and Alireza Hasanzadeh are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghozy, S., Amoukhteh, M., Hasanzadeh, A. et al. Net water uptake as a predictive neuroimaging marker for acute ischemic stroke outcomes: a meta-analysis. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10599-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10599-6