Abstract
Objectives
The performance of positron emission tomography/computed tomography (PET/CT) for the prediction of ypN2 disease in non-small cell lung cancer (NSCLC) after neoadjuvant chemoimmunotherapy has not been reported. This multicenter study investigated the utility of PET/CT to assess ypN2 disease in these patients.
Methods
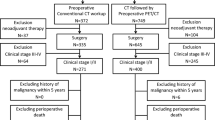
A total of 181 consecutive patients (chemoimmunotherapy = 86, chemotherapy = 95) at four institutions were enrolled in this study. Every patient received a PET/CT scan prior to surgery and complete resection with systematic nodal dissection. The diagnostic performance was evaluated through area under the curve (AUC). Kaplan–Meier method and Cox analysis were performed to identify the risk factors affecting recurrences.
Results
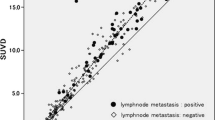
The sensitivity, specificity, and accuracy of PET/CT for ypN2 diseases were 0.667, 0.835, and 0.779, respectively. Therefore, the AUC was 0.751. Compared with the false positive cases, the mean value of max standardized uptake value (SUVmax) (6.024 vs. 2.672, p < 0.001) of N2 nodes was significantly higher in true positive patients. Moreover, the SUVmax of true positive (7.671 vs. 5.976, p = 0.365) and false (2.433 vs. 2.339, p = 0.990) positive cases were similar between chemoimmunotherapy and chemotherapy, respectively. Survival analysis proved that pathologic N (ypN) 2 patients could be stratified by PET/CT-N2(+ vs. -) for both chemoimmunotherapy (p = 0.023) and chemotherapy (p = 0.010).
Conclusions
PET/CT is an accurate and non-invasive test for mediastinal restaging of NSCLC patients who receive neoadjuvant chemoimmunotherapy. The ypN2 patients with PET/CT-N2( +) are identified as an independent prognostic factor compared with PET/CT-N2(-).
Clinical relevance statement
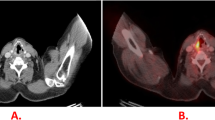
Imaging with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) plays an integral role during disease diagnosis, staging, and therapeutic response assessments in patients with NSCLC. PET/CT could be an effective non-invasive tool for predicting ypN2 diseases after neoadjuvant chemoimmunotherapy.
Key Points
• PET/CT could serve as an effective non-invasive tool for predicting ypN2 diseases.
• The ypN2 patients with PET/CT-N2( +) were a strong and independent prognostic factor.
• The application of PET/CT for restaging should be encouraged in clinical practice.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- AUC:
-
Area under the curves
- CI:
-
Confidence interval
- CR:
-
Complete response
- EBUS-TBNA:
-
Endobronchial ultrasound–guided transbronchial needle aspiration
- EFS:
-
Event-free survival
- IASLC:
-
International Association for the Study of Lung Cancer
- ICIs:
-
Immune checkpoint inhibitors
- MPR:
-
Major pathologic response
- NCCN:
-
National Comprehensive Cancer Network
- NIF:
-
Nodal immune flare
- NPV:
-
Negative predictive value
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- pCR:
-
Pathologic complete response
- PD:
-
Progressive disease
- PET/CT:
-
Positron emission tomography/computed tomography
- pN:
-
Pathologic N
- PPV:
-
Positive predictive value
- PR:
-
Partial response
- RECIST:
-
The Response Evaluation Criteria In Solid Tumors
- RFS:
-
Recurrence-free survival
- ROC:
-
Receiver operating characteristic
- SD:
-
Stable disease
References
Rocco G, Nason K, Brunelli A, Varela G, Waddell T, Jones DR (2016) Management of stage IIIA (N2) non-small-cell lung cancer: a transatlantic perspectivedagger. Eur J Cardiothorac Surg 49:1025–1027
Asamura H, Chansky K, Crowley J et al (2015) The International Association for the Study of Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM Classification for Lung Cancer. J Thorac Oncol 10:1675–1684
Forde PM, Chaft JE, Smith KN et al (2018) Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 378:1976–1986
Provencio M, Nadal E, Insa A et al (2020) Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:1413–1422
Shu CA, Gainor JF, Awad MM et al (2020) Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:786–795
Spicer J, Wang CL, Tanaka F et al (2021) Surgical outcomes from the phase 3 CheckMate 816 trial: nivolumab (NIVO) plus platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol 39(15):8503–8503. https://doi.org/10.1200/JCO.2021.39.15_suppl.8503
Provencio M, Serna-Blasco R, Nadal E et al (2022) Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial). J Clin Oncol 40:2924–2933
Wu J, Hou L, E H, et al (2022) Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer 165:115–123
Jiang L, Huang J, Jiang S et al (2021) The surgical perspective in neoadjuvant immunotherapy for resectable non-small cell lung cancer. Cancer Immunol Immunother 70:2313–2321
Liang W, Cai K, Chen C et al (2020) Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 9:2696–2715
De Leyn P, Stroobants S, De Wever W et al (2006) Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 24:3333–3339
Herth FJ, Annema JT, Eberhardt R et al (2008) Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 26:3346–3350
Turgeon GA, Iravani A, Akhurst T et al (2019) What (18)F-FDG PET response-assessment method best predicts survival after curative-intent chemoradiation in non-small cell lung cancer: EORTC, PERCIST, Peter Mac Criteria, or Deauville Criteria? J Nucl Med 60:328–334
Eze C, Schmidt-Hegemann NS, Sawicki LM et al (2021) PET/CT imaging for evaluation of multimodal treatment efficacy and toxicity in advanced NSCLC-current state and future directions. Eur J Nucl Med Mol Imaging 48:3975–3989
Tao X, Li N, Wu N et al (2020) The efficiency of (18)F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging 47:1209–1219
Zhao ZR, Yang CP, Chen S et al (2021) Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology 10:1996000
Cascone T, Weissferdt A, Godoy MCB et al (2021) Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat Commun 12:5045
Rami-Porta R, Wittekind C, Goldstraw P (2020) Complete resection in lung cancer surgery: from definition to validation and beyond. J Thorac Oncol 15:1815–1818
Postmus PE, Kerr KM, Oudkerk M et al (2017) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv1–iv211
Xie D, Wang H, Fei K et al (2016) Single-port video-assisted thoracic surgery in 1063 cases: a single-institution experiencedagger. Eur J Cardiothorac Surg 49(Suppl 1):i31-36
Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P (2009) The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 4:568–577
Travis WD, Dacic S, Wistuba I et al (2020) IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol 15:709–740
Pataer A, Weissferdt A, Vaporciyan AA et al (2021) Evaluation of pathologic response in lymph nodes of patients with lung cancer receiving neoadjuvant chemotherapy. J Thorac Oncol 16:1289–1297
Uprety D, Mandrekar SJ, Wigle D, Roden AC, Adjei AA (2020) Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J Thorac Oncol 15:1281–1297
O’Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL (2019) The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res 25:5743–5751
Cascone T, Provencio M, Sepesi B et al (2020) Checkmate 77T: a phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. 38:TPS9076-TPS9076
Fernando HC, Yang J, Ferraro GL, Keller SM (2018) Randomized, double-blind phase 3 study evaluating neoadjuvant platinum-based chemotherapy with perioperative pembrolizumab or placebo in resectable stage IIB or IIIA NSCLC: KEYNOTE-671. J Clin Oncol 36(15):TPS8583–TPS8583. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS8583
Peters S, Kim AW, Solomon B et al (2019) IMpower030: phase III study evaluating neoadjuvant treatment of resectable stage II-IIIB non-small cell lung cancer (NSCLC) with atezolizumab (atezo) + chemotherapy. Ann Oncol 30:ii30. https://doi.org/10.1093/annonc/mdz064.014
Cascone T, William WN Jr, Weissferdt A et al (2021) Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 27:504–514
Champion L, Lerebours F, Alberini JL et al (2015) 18F-FDG PET/CT to predict response to neoadjuvant chemotherapy and prognosis in inflammatory breast cancer. J Nucl Med 56:1315–1321
Valkema MJ, van der Wilk BJ, Eyck BM et al (2021) Surveillance of clinically complete responders using serial (18)F-FDG PET/CT scans in patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Nucl Med 62:486–492
Hua J, Li L, Liu L, Liu Q, Liu Y, Chen X (2021) The diagnostic value of metabolic, morphological and heterogeneous parameters of 18F-FDG PET/CT in mediastinal lymph node metastasis of non-small cell lung cancer. Nucl Med Commun 42:1247–1253
Kirchner J, Sawicki LM, Nensa F et al (2019) Prospective comparison of (18)F-FDG PET/MRI and (18)F-FDG PET/CT for thoracic staging of non-small cell lung cancer. Eur J Nucl Med Mol Imaging 46:437–445
Kubota K, Murakami K, Inoue T et al (2011) Additional value of FDG-PET to contrast enhanced-computed tomography (CT) for the diagnosis of mediastinal lymph node metastasis in non-small cell lung cancer: a Japanese multicenter clinical study. Ann Nucl Med 25:777–786
Lv YL, Yuan DM, Wang K et al (2011) Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 6:1350–1358
De Leyn P, Dooms C, Kuzdzal J et al (2014) Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 45:787–798
Ripley RT, Suzuki K, Tan KS et al (2016) Postinduction positron emission tomography assessment of N2 nodes is not associated with ypN2 disease or overall survival in stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 151:969–977, 979 e961-963
Jin P, Li J, Meng Y et al (2021) PET/CT metabolic patterns in systemic immune activation: a new perspective on the assessment of immunotherapy response and efficacy. Cancer Lett 520:91–99
Liu X, Sun W, Wu J et al (2021) Major pathologic response assessment and clinical significance of metastatic lymph nodes after neoadjuvant therapy for non-small cell lung cancer. Mod Pathol 34:1990–1998
Zappasodi R, Serganova I, Cohen IJ et al (2021) CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours. Nature 591:652–658
Bader JE, Voss K, Rathmell JC (2020) Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell 78:1019–1033
Deng J, Zhong Y, Wang T et al (2022) Lung cancer with PET/CT-defined occult nodal metastasis yields favourable prognosis and benefits from adjuvant therapy: a multicentre study. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-022-05690-3
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2500904 and 2021YFC2500905), Shanghai Municipal Health Commission (202040322), and Shanghai Hospital Development Center (SHDC22021217).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
Dr. Chang Chen has been identified as the guarantor, taking responsibility for the content of the manuscript, including the data and analysis.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
One of the authors has significant statistical expertise.
Informed Consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
• retrospective
• cross sectional study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., E, H., Huang, J. et al. Clinical utility of [18F]FDG PET/CT in the assessment of mediastinal lymph node disease after neoadjuvant chemoimmunotherapy for non-small cell lung cancer. Eur Radiol 33, 8564–8572 (2023). https://doi.org/10.1007/s00330-023-09910-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09910-8