Abstract
Objectives
To test if tumour changes measured using combination of diffusion-weighted imaging (DWI) MRI and FDG-PET/CT performed serially during radiotherapy (RT) in mucosal head and neck carcinoma can predict treatment response.
Methods
Fifty-five patients from two prospective imaging biomarker studies were analysed. FDG-PET/CT was performed at baseline, during RT (week 3), and post RT (3 months). DWI was performed at baseline, during RT (weeks 2, 3, 5, 6), and post RT (1 and 3 months). The ADCmean from DWI and FDG-PET parameters SUVmax, SUVmean, metabolic tumour volume (MTV), and total lesion glycolysis (TLG) were measured. Absolute and relative change (%∆) in DWI and PET parameters were correlated to 1-year local recurrence. Patients were categorised into favourable, mixed, and unfavourable imaging response using optimal cut-off (OC) values of DWI and FDG-PET parameters and correlated to local control.
Results
The 1-year local, regional, and distant recurrence rates were 18.2% (10/55), 7.3% (4/55), and 12.7% (7/55), respectively. ∆Week 3 ADCmean (AUC 0.825, p = 0.003; OC ∆ > 24.4%) and ∆MTV (AUC 0.833, p = 0.001; OC ∆ > 50.4%) were the best predictors of local recurrence. Week 3 was the optimal time point for assessing DWI imaging response. Using a combination of ∆ADCmean and ∆MTV improved the strength of correlation to local recurrence (p ≤ 0.001). In patients who underwent both week 3 MRI and FDG-PET/CT, significant differences in local recurrence rates were seen between patients with favourable (0%), mixed (17%), and unfavourable (78%) combined imaging response.
Conclusions
Changes in mid-treatment DWI and FDG-PET/CT imaging can predict treatment response and could be utilised in the design of future adaptive clinical trials.
Clinical relevance statement
Our study shows the complementary information provided by two functional imaging modalities for mid-treatment response prediction in patients with head and neck cancer.
Key Points
•FDG-PET/CT and DWI MRI changes in tumour during radiotherapy in head and neck cancer can predict treatment response.
•Combination of FDG-PET/CT and DWI parameters improved correlation to clinical outcome.
•Week 3 was the optimal time point for DWI MRI imaging response assessment.
Similar content being viewed by others
Introduction
Definitive radiotherapy with or without concurrent systemic treatment is a standard organ-preserving treatment for locally advanced mucosal head and neck squamous cell carcinoma. Following conventional treatment, a proportion of patients still experience locoregional tumour recurrence causing significant morbidity or death. Further, in those who are cured, the treatment is associated with significant acute and late toxicities. Commonly used tumour stage (TNM), smoking history, and HPV status provide prognostic information but have failed to be useful as predictive markers in risk-adaptation studies [1,2,3]. A reliable quantitative biomarker that can predict outcomes is therefore needed to guide adapted intensification or de-intensification of treatment based on response.
Functional imaging can characterise tumour biology using quantitative measures that can act as an early surrogate marker for treatment response. Diffusion-weighted imaging MRI (DWI) and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET/CT) have the advantage of non-invasively monitoring the tumour changes via serial imaging during radiotherapy. Correlational data suggest that DWI and FDG-PET/CT provide complementary biological information [4, 5]. To date, most imaging biomarker studies in head and neck cancer have analysed pre-treatment images. Changes in functional imaging performed during treatment have been shown to be a better biomarker of treatment response compared to pre-treatment imaging [6,7,8,9,10]. Studies analysing mid-treatment images have been limited by utilising single imaging modality, small sample size, and non-uniform methodologies. Timing of mid-treatment imaging also significantly impacts response assessment but the optimal time point for assessing response also remains unanswered.
Therefore, there remains a need for a prospective multimodality imaging study to assess the utility of quantifying mid-treatment tumour changes that predict response to radiotherapy in head and neck cancer.
The aim of this study was to evaluate if tumour changes measured using a combination of DWI and FDG-PET/CT performed serially during radiotherapy in head and neck cancer can be used to predict for treatment response. We also sought to find the optimal time point for assessing DWI in future imaging biomarker studies.
Materials and methods
Study design
Patients with newly diagnosed, biopsy-proven, non-metastatic mucosal head and neck squamous cell carcinoma treated with definitive radiotherapy with or without concurrent systemic therapy from two independent prospective quantitative imaging biomarker studies were evaluated. Patients in study 1 were recruited from June 2014 to May 2015. Patients in the subsequent study 2 were recruited from May 2016 to October 2019 [11]. Local research ethics committee approval was provided for both studies and all patients provided written informed consent.
All patients were evaluated and reviewed by a multidisciplinary head and neck team. Patients were treated using a simultaneous integrated boost intensity-modulated radiation therapy technique over 35 fractions. Radiotherapy treatment volumes were defined using consensus international guidelines and underwent a stringent peer review process [12].
Patients in both studies underwent serial multiparametric MRI (DWI, T2) imaging before, during (weeks 2, 3, 5, 6), and following completion (1 month and 3 months) of radiotherapy. Patients also underwent 18F-FDG-PET/CT before and during (week 3) radiotherapy. The imaging timeline utilised in both studies is shown in Fig. 1. Treatment response was evaluated with post-treatment 18F-FDG-PET/CT and clinical examination including nasoendoscopy. Recurrences were confirmed histologically or via imaging following discussion at a multidisciplinary head and neck meeting.
The imaging results were compared with the 1-year local (primary tumour) recurrence measured from time of diagnosis. Secondary outcomes included 1-year regional (nodal) recurrence, distant (non-regional nodes and visceral metastasis) recurrence, and death.
Image acquisition
MRI was performed on a dedicated 3.0-T scanner (MAGNETOM® Skyra; Siemens Healthcare). Sequences obtained were DWI, using a readout-segmented EPI technique (RESOLVE; Siemens Healthineers), using b = 50 and 800 s mm−2 with signal averages set to 1 and 3, respectively. Morphological axial T2-weighted and T1-weighted images were also acquired. Details of the imaging technique have been described previously [11].
PET studies were acquired in radiotherapy treatment position on a GE Discovery™-710 time-of-flight positron emission tomography (PET)-CT (GE Healthcare, Waukesha, MI). FDG-PET/CT imaging was performed in accordance with EANM clinical guidelines [13]. Patients received 4.1 MBq kg−1 of [18F]FDG after at least 4 h of fasting. The average blood sugar level was 5.7 ± 1.2 mmol/L (range 3.3–9.6 mmol/L). The staging and all sequential scans were performed on the same scanner with the same acquisition and reconstruction protocols. Details of the imaging technique have been described previously [6].
Image analysis
The primary region of interest (ROI) captured was the primary tumour. To aid accurate delineation of tumour, clinical examination and nasoendoscopic findings were provided in conjunction with patient images. All PET and MRI images were viewed and had ROIs delineated using commercial image visualisation and delineation software (MIM Software Inc.). DICOM images containing ROIs were subsequently analysed using open-source PyRadiomics software (v2.2.0) [14].
The tumour volumes were delineated by a radiation oncologist (Y.T.) in consensus with a consultant radiologist (D.M.) and nuclear medicine physician (P.L.) for each imaging modality at every time point. Due to spatially non-linear distortions associated with DWI, ROIs were manually defined on ADCmaps while primarily referencing to the co-registered low b-value diffusion-weighted images (b50) as per consensus recommendations and methodology performed previously [10, 15]. Co-registered T2-weighted images were also viewed at the same time to aid delineation. The mean values of apparent diffusion coefficient (ADCmean) were calculated from all ROIs. FDG-PET-derived metabolic tumour volume (MTV) was calculated using the PETedge tool of MIM software, a semi-automated gradient method. Other PET parameters including SUVmax, SUVmean, and total lesion glycolysis (TLG = SUVmean × MTV) were also calculated for all ROIs. We have previously found anatomical tumour volumes on T2 MRI and CT images and their relative change were not correlated to clinical outcomes; hence, they were not included in this analysis.
The percentage change (∆) in ADC and FDG-PET/CT imaging parameters from baseline was calculated for each time point, defined as ∆ = (value − baseline) / baseline × 100%.
Statistical analysis
The changes in PET and DWI parameters from baseline were compared using the Wilcoxon signed-rank test.
The absolute value and change (D) in parameters were compared to treatment outcomes using the Mann-Whitney U test. For parameters with predictive value, receiver operating characteristic (ROC) analysis was performed using the area under the curve (AUC) as an index of accuracy to differentiate between multiple predictive parameters. Optimal cut-off values for analysis were derived from the ROC curves aiming for best sensitivity and specificity by applying the Youden index [16]. Local recurrence-free survival (LRFS), regional recurrence-free survival (RFS), distant metastatic recurrence-free survival (DMRFS), and overall survival (OS) curves were estimated using Kaplan-Meier analysis and compared using the log-rank (Mantel-Cox) test.
Combined modality response
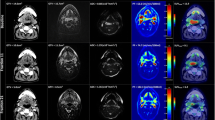
To determine the utility of combining multiple imaging modalities, the most accurate FDG-PET/CT parameter and DWI parameter that correlated to local recurrence (highest ROC value in AUC analysis) were chosen for subsequent analysis. Optimum cut-off values from the two parameters were used to determine patient’s response in each imaging modality. Patients were subsequently divided into three groups based on those that had favourable response in both FDG-PET/CT and DWI imaging; mixed response (good PET and poor DWI, or poor PET and good DWI); or poor response in both imaging modalities (for a representative example of three groups, see Fig. 4). The three groups were compared to local recurrence status using the Pearson chi-squared test, using Cramer’s V test to check the strength of association to determine the utility of combining multiple parameters in improving predictive ability.
A pre-specified split-sample internal validation using the two independent enrolling studies was undertaken to explore the stability of results in combining multimodality parameters (described in detail in Supplementary Material).
The data were analysed using SPSS statistical software (version 24.0; IBM Corp). Statistical significance was considered p < 0.05.
Results
Patient characteristics
Fifty-five patients were available for analysis: 30 patients in study 1 and 25 patients in study 2. Patient, tumour, and treatment details are summarised in Table 1. The median follow-up was 34.0 months (range 4–68), with a minimum follow-up of 12 months for patients who were alive.
The 1-year local, regional, and distant recurrence rates were 18.2% (10/55), 7.3% (4/55), and 12.7% (7/55), respectively. At the time of analysis, 18 patients had died, 13 from recurrent cancer and 5 from other causes.
Primary tumour analysis
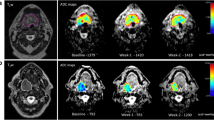
The number of DWI tumour ROIs analysed at individual time points were 55(baseline), 47 (week 2), 42 (week 3), 37 (week 5), 34 (week 6), 36 (11 weeks), and 38 (19 weeks). The number of FDG-PET/CT tumour ROIs analysed at individual time points were 55 (baseline) and 53 (week 3). The mean of primary tumour ADCmean increased during radiotherapy and plateaued at week 5 (see Fig. 2A). For the entire population, the mean FDG-PET/CT parameters reduced at week 3 compared to baseline (see Fig. 3A). There was a statistically significant difference in the absolute ADCmean and FDG-PET/CT parameter values at each time point relative to baseline.
Correlation of primary tumour parameters to local recurrence
Patients with local recurrence had a higher ADCmean value at baseline compared to those without. However, this did not reach statistical significance (p = 0.135). Absolute values of ADCmean at all time points did not correlate to local recurrence. Patients with local recurrence had a lower rise in ADCmean value over time (Supplementary Figure 1). The relative change in ADCmean (∆ADCmean) at all time points except week 5 correlated to local recurrence (see Fig. 2B). The differences in the mean of absolute ADCmean values and the ∆ADCmean based on local recurrence status for the entire group are provided in Table 2. Relative change in ADCmean at week 3 (∆Week 3 ADCmean) was the best predictor of local recurrence (AUC 0.825, p = 0.003) (Supplementary Figure 2). Optimal cut-off values of ∆Week 3 ADCmean for predicting local recurrence was < 24.4% rise in ADCmean, resulting in 89% sensitivity, 88% specificity, PPV 97%, and NPV 67%.
No absolute values of SUVmax, SUVmean, MTV, and TLG at baseline correlated to local recurrence. ∆MTV (p = 0.001) and closely related ∆TLG (p = 0.005) correlated to local recurrence (see Fig. 3B). Difference in absolute values and relative change in FDG-PET/CT parameters based on local recurrence status are provided in Table 2. Relative change in MTV at week 3 (∆MTV) was the best predictor of local recurrence (AUC 0.833, p = 0.001). Optimal cut-off of ∆MTV for predicting local recurrence was < 50.4% drop in MTV; resulting in 91% sensitivity, 64% specificity, PPV 64%, and NPV 96%.
To test the primary hypothesis of the added value of combining DWI and FDG-PET/CT parameters, ∆ADCmean and ∆MTV at week 3 were chosen to define response in DWI and FDG-PET/CT imaging. Using optimal cut-off value of ∆Week 3 ADCmean (> 24.4%) and ∆MTV (< 50.4%) to define response in each imaging modality, 20 patients were defined as favourable responders (> 24.4% ∆Week 3 ADCmean, and > 50.4% ∆MTV), 9 patients were defined as unfavourable responders (< 24.4% ∆Week 3 ADCmean, and <50.4% ∆MTV), and 12 patients were defined as mixed responders. In favourable responders, no patients (0/20) had local recurrence. In mixed responders, 17% (2/12) had local recurrence. In unfavourable responders, 78% (7/9) had local recurrence (for a representative example of three groups, see Fig. 4). The Kaplan-Meier analysis of local recurrence stratified by the above three response groups (subgroups) is shown in Fig. 5. Using a combination of ∆ADCmean and ∆MTV improved the strength of correlation to local recurrence (Cramer’s V 0.736, p ≤ 0.001, Pearson chi-squared test) compared to using individual parameter alone (Cramer’s V 0.693, 0.586).
As a part of pre-specified split sample internal validation, ∆Week 3 ADCmean and ∆MTV were selected for analysis in patients in study 1 as a training dataset (n = 30). Successful internal validation was performed on the testing dataset of patients in study 2 (n = 25) confirming use of combined primary tumour DWI and FDG-PET/CT parameters in predicting 1-year local recurrence (see Supplementary Material for details).
Correlation of primary tumour parameters to regional recurrence, distant recurrence, and death
Only baseline ADCmean value correlated to death (AUC 0.685, p = 0.027) (Supplementary Table 1). Using an optimal cut-off value of 1301 × 10−6mm2s−1 for baseline primary tumour ADCmean resulted in 2-year OS of 82% vs 65% (log rank p = 0.004) (see Supplementary Figure 3).
Baseline, week 3, and relative change in MTV and TLG at week 3 (∆MTV, ∆TLG) correlated to death, which occurred as a result of local tumour recurrence in majority of cases (Supplementary Table 1). Absolute value of TLG at week 3 was the best predictor for death (AUC 0.742, p = 0.005). Using an optimal cut-off value of 16.0 g resulted in 2-year OS of 96% vs 45%, p ≤ 0.001) (see Supplementary Figure 4).
Discussion
Our study represents the largest published prospective mid-treatment imaging biomarker series utilising DWI and FDG-PET/CT scans performed during radiotherapy in head and neck squamous cell carcinoma (HNSCC). We identified functional changes in tumour during definitive radiotherapy in HNSCC using FDG-PET/CT and DWI which can be used to predict treatment response. Using a novel method, our data suggest that combination of multiple functional imaging parameters improves correlation to local tumour control and could potentially be used to identify sub-groups of patients for future risk-adapted radiotherapy clinical trials. We also identified week 3 mid-treatment as an optimal time point to perform DWI imaging to assess primary tumour response in future imaging studies.
We showed that primary tumour ADCmean increased and plateaued around week 5 during radiotherapy. Change in week 3 primary tumour ADCmean had the strongest correlation to local control. Based on our data, greater percentage change in ADCmean (∆ > 24.4%) was highly predictive of good treatment response leading to local tumour control following radiotherapy. Studies by Motaba et al and Ghany et al also found ∆ADCmean at week 3 of 24% and 20% respectively correlated to tumour control [18, 19]. The higher rise in ADCmean during radiotherapy in good responders is explained by greater tumour cell loss. However, as radiotherapy progresses, increasing treatment-related oedema would also alter the ADC signal [17]. Week 3 likely represents the optimal time point for assessing treatment response due to higher ratio of signal from tumour prior to onset of noise from radiotherapy-related oedema [18]. Previous studies looking to identify the optimal time point for DWI response assessment were limited to 2 to 3 mid-treatment imaging time points [17,18,19,20,21]. The size of our study and extensive serial time points for DWI treatment response assessment adds considerably to the current published literature.
FDG-PET/CT imaging measures functional aspects of the tumour that are known to predict for poor radiotherapy response such as high tumour cellularity, metabolism, and/or hypoxia [19,20,21]. We have shown that change in primary tumour MTV at week 3 had the strongest correlation to local tumour control. Previous studies also show that FDG-PET/CT-based functional tumour volume (MTV) outperforms anatomical data for treatment response prediction [22, 23]. Based on our data, a greater percentage decrease in MTV (∆ > 50.4%) in primary tumour was highly predictive of tumour control. Our results confirm findings from previous studies that changes in FDG-PET/CT metabolic parameters are better treatment response markers compared to absolute values [22, 24]. Studies by Myo et al and Pollom et al have shown that ∆TLG from mid-treatment FDG-PET/CT can predict clinical outcomes [6, 28]. The studies utilised differing methodologies to delineate the MTV and hence limits direct comparison. Aside from non-standardised imaging protocol, performance of FDG-PET/CT-based parameters can also be limited by confounding influence of radiotherapy-induced inflammation/necrosis [24]. Hence, a rationale exists for utilisation of concomitant functional MRI imaging to provide additional discriminatory information to improve the prediction of treatment response.
We showed that utilisation of multiple functional imaging modalities improves incremental predictive performance for assessing tumour response following radiotherapy compared to individual imaging modality. FDG-PET/CT parameter provided good sensitivity but only moderate specificity due to treatment-related changes. The addition of DWI parameter improved the specificity and hence the predictive performance. The majority of published imaging biomarker studies have focused on a single imaging modality, predominantly CT, fMRI, and to a lesser extent FDG-PET and 18F-MISO PET. Multimodality functional imaging offers the advantage of measuring different tumour characteristics and improving the performance of individual imaging modalities in predicting treatment response [4, 5, 25, 26]. Wong et al have published the only other study containing 35 patients correlating changes in MRI and FDG-PET/CT imaging parameters during radiotherapy to clinical outcomes in HNSCC [10]. They found individual fMRI and FDG-PET/CT features that successfully differentiated responders from non-responders based on a surrogate endpoint of 3-month post-treatment imaging response.
Using optimal cut-off values of the highest performing FDG-PET/CT (week 3 ∆MTV) and DWI (week 3 ∆ADCmean) parameter, we were able to separate HNSCC patients into three risk sub-groups based on their early response during radiotherapy. This is potentially more flexible and accurate than traditional staging with AJCC as it accounts for tumour biology and treatment sensitivity. This stratification could be utilised in future mid-treatment risk-adaptation clinical trials. Patients with good FDG-PET/CT and DWI response had low rate of local failure (0%) and could be considered for de-escalation, e.g. radiotherapy dose or volume reduction, to reduce treatment-related toxicities. Patients with poor FDG-PET/CT and DWI response had high rates of local failure (78%) and could be considered for treatment intensification, e.g. radiotherapy dose boost, acceleration, use of radiosensitisers, or bail-out surgery. Our approach of risk stratifying patients is novel and hypothesis generating requiring external validation prior to clinical application. There are currently no published HNSCC studies that have utilised mid-treatment week 3 DWI and FDG-PET/CT imaging to allow external validation. Appreciating the limitation of the data available, a pre-specified split sample internal validation using patients from the two temporally separate studies was successfully undertaken to explore the stability of our results.
Strengths of our study include the large number of patients and standardised imaging time points for response assessment. Patients in our study were prospectively recruited, underwent standardised imaging protocol, and had reproducible consensus-based ROI delineation using best available guidelines [6, 10, 11, 14, 15, 27, 28]. We also correlated imaging parameters with long-term clinical outcomes rather than surrogate endpoints such as early post-treatment imaging. There are a few limitations of our study. Ours was a single-institutional study with all serial imaging undertaken on the same dedicated MRI and PET machine, and hence extrapolation of our results without additional standardisation quality assurance activities or utilisation of our methodology should be done with caution [29, 30]. We also noted attrition of patients undergoing MRI imaging as radiotherapy progressed due to poor patient tolerability; however, ∆Week 3 ADCmean remained significant for assessing treatment response when analysing only patients who underwent serial DWI imaging at every time point. Patients included in our series represent the commonly seen heterogeneous sub-sites of mucosal head and neck cancer treated with radiotherapy. Therefore, our results should be replicated in a larger multi-institutional setting with a more homogeneous population prior to clinical application. In a multi-institutional setting, additional quality assurance to harmonise image quality is also required. However, we believe that parameters measuring relative change from baseline if done on the same scanner provides greater reproducibility due to auto-normalisation of measurements compared to using a single static value.
Conclusion
Our study highlights the importance of changes in mid-treatment DWI and FDG-PET/CT imaging in predicting treatment response. We identified a combination of change in week 3 DWI (> 24.4% ∆ADCmean) and FDG-PET/CT (> 50.4% ∆MTV) imaging parameters performed during radiotherapy that could be utilised in the design of future risk-adapted clinical trials in HNSCC.
Abbreviations
- ∆:
-
Change in value = (value − baseline) / baseline × 100%
- ADCmean :
-
Mean value of apparent diffusion coefficient
- AUC:
-
Area under the (receiver operating characteristic) curve
- DMRFS:
-
Distant metastatic recurrence-free survival
- DWI:
-
Diffusion-weighted imaging MRI
- FDG-PET:
-
18F-Fluorodeoxyglucose-positron emission tomography
- fMRI:
-
Functional MRI
- HNSCC:
-
Mucosal head and neck squamous cell carcinoma
- LRFS:
-
Local recurrence-free survival
- MTV:
-
Metabolic tumour volume
- OC:
-
Optimal cut-off value from receiver operating characteristic analysis
- OS:
-
Overall survival
- RFS:
-
Regional/nodal recurrence-free survival
- ROI:
-
Region of interest, primary tumour contour delineation
- RT:
-
Radiotherapy
- TLG:
-
Total lesion glycolysis
- TNM:
-
Tumour, node, and metastases staging classification
References
Mehanna H, Robinson M, Hartley A et al (2019) Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393:51–60
Gillison ML, Trotti AM, Harris J et al (2019) Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393:40–50
Nichols AC, Theurer J, Prisman E et al (2019) Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 20(10):1349–1359
Teng F, Aryal M, Lee J et al (2018) Adaptive boost target definition in high-risk head and neck cancer based on multi-imaging risk biomarkers. Int J Radiat Oncol Biol Phys 102(4):969–977
Martens RM, Koopman T, Lavini C et al (2021) Multiparametric functional MRI and (18)F-FDG-PET for survival prediction in patients with head and neck squamous cell carcinoma treated with (chemo)radiation. Eur Radiol 31(2):616–628
Min M, Lin P, Lee MT et al (2015) Prognostic role of metabolic parameters of (18)F-FDG PET-CT scan performed during radiation therapy in locally advanced head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging 42(13):1984–1994
Chen SW, Hsieh TC, Yen KY et al (2014) Interim FDG PET/CT for predicting the outcome in patients with head and neck cancer. Laryngoscope 124(12):2732–2738
King AD, Mo FK, Yu KH et al (2010) Squamous cell carcinoma of the head and neck: diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol 20(9):2213–2220
Kim S, Loevner L, Quon H et al (2009) Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res 15(3):986–994
Wong KH, Panek R, Dunlop A et al (2018) Changes in multimodality functional imaging parameters early during chemoradiation predict treatment response in patients with locally advanced head and neck cancer. Eur J Nucl Med Mol Imaging 45(5):759–767
Rumley CN, Lee MT (2017) Holloway L, et al Multiparametric magnetic resonance imaging in mucosal primary head and neck cancer: a prospective imaging biomarker study. BMC Cancer 17(1):475
Gregoire V, Ang K, Budach W et al (2014) Delineation of the neck node levels for head and neck tumours: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 110(1):172–181
Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42(2):328–354
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77(21):104–107
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11(2):102–125
Fluss R, Faraggi D, Reiser B (2005) Estimation of the Youden Index and its associated cutoff point. Biom J 47(4):458–472
Cao Y, Tseng CL, Balter JM, Teng F, Parmar HA, Sahgal A (2017) MR-guided radiation therapy: transformative technology and its role in the central nervous system. Neuro Oncol; https://doi.org/10.1093/neuonc/nox006
Martens RM, Noij DP, Ali M et al (2019) Functional imaging early during (chemo)radiotherapy for response prediction in head and neck squamous cell carcinoma; a systematic review. Oral Oncol 88:75–83
Herholz K, Pietrzyk U, Voges J et al (1993) Correlation of glucose consumption and tumour cell density in astrocytomas. A Stereotact PET Stud J Neurosurg 79(6):853–858
Rajendran JG, Mankoff DA, O’Sullivan F et al (2004) Hypoxia and glucose metabolism in malignant tumours: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10(7):2245–2252
Jain VK, Kalia VK, Sharma R, Maharajan V, Menon M (1985) Effects of 2-deoxy-D-glucose on glycolysis, proliferation kinetics and radiation response of human cancer cells. Int J Radiat Oncol Biol Phys 11(5):943–950
Pollom EL, Song J, Durkee BY et al (2016) Prognostic value of midtreatment FDG-PET in oropharyngeal cancer. Head Neck 38(10):1472–1478
Kong FS, Li L, Wang W et al (2019) Greater reduction in mid-treatment FDG-PET volume may be associated with worse survival in non-small cell lung cancer. Radiother Oncol 132:241–249
Lin P, Min M, Lee M et al (2017) Nodal parameters of FDG PET/CT performed during radiotherapy for locally advanced mucosal primary head and neck squamous cell carcinoma can predict treatment outcomes: SUVmean and response rate are useful imaging biomarkers. Eur J Nucl Med Mol Imaging 44(5):801–811
Preda L, Conte G, Bonello L et al (2016) Combining standardized uptake value of FDG-PET and apparent diffusion coefficient of DW-MRI improves risk stratification in head and neck squamous cell carcinoma. Eur Radiol 26(12):4432–4441
Houweling AC, Wolf AL, Vogel WV et al (2013) FDG-PET and diffusion-weighted MRI in head-and-neck cancer patients: implications for dose painting. Radiother Oncol 106(2):250–254
Becker M, Zbaren P, Casselman JW, Kohler R, Dulguerov P, Becker CD (2008) Neoplastic invasion of laryngeal cartilage: reassessment of criteria for diagnosis at MR imaging. Radiology 249(2):551–559
Jager EA, Ligtenberg H, Caldas-Magalhaes J et al (2016) Validated guidelines for tumour delineation on magnetic resonance imaging for laryngeal and hypopharyngeal cancer. Acta Oncol 55(11):1305–1312
deSouza NM, Winfield JM, Waterton JC et al (2018) Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: current considerations and future perspectives. Eur Radiol 28(3):1118–1131
Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le RC (2011) Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging 38(7):1191–1202
Funding
Open Access funding enabled and organised by CAUL and its Member Institutions The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Yuvnik Trada.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in initial trial protocols and preliminary analysis of technical data without clinical results.
Methodology
•prospective
•diagnostic or prognostic study
•performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 261 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trada, Y., Keall, P., Jameson, M. et al. Changes in serial multiparametric MRI and FDG-PET/CT functional imaging during radiation therapy can predict treatment response in patients with head and neck cancer. Eur Radiol 33, 8788–8799 (2023). https://doi.org/10.1007/s00330-023-09843-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09843-2