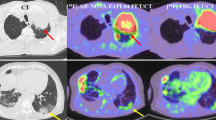
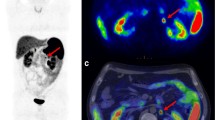
Abstract
Objectives
To evaluate the prognostic value of TLR from PET/CT in patients with resection margin-negative stage IB and IIA non-small cell lung cancer (NSCLC) and compare high-risk factors necessitating adjuvant treatment (AT).
Methods
Consecutive FDG PET/CT scans performed for the initial staging of NSCLC stage IB and IIA were retrospectively reviewed. The maximum standardized uptake value (SUVmax) of the primary tumor and mean SUV of the liver were acquired. The tumor-to-liver SUV ratio (TLR) was also calculated. Charts were reviewed for basic patient characteristics and high-risk factors for considering AT (poor differentiation, visceral pleura invasion, vascular invasion, tumors > 4 cm, and wedge resection). Statistical analysis was performed using Cox regression analysis and the Kaplan–Meier method.
Results
Of the 112 patients included, 15 (13.4%) died, with a median overall survival (OS) of 43.8 months. Twenty-two patients (19.6%) exhibited recurrence, with median disease-free survival (DFS) of 36.0 months. In univariable analysis, pathology, poor differentiation, and TLR were associated with shorter DFS and OS. In multivariable analysis, TLR (hazard ratio [HR] = 1.263, p = 0.008) and differentiation (HR = 3.087, p = 0.012) were associated with shorter DFS. Also, TLR (HR = 1.422, p < 0.001) was associated with shorter OS.
Conclusion
TLR from FDG PET/CT was an independent prognostic factor for recurrence and survival. PET parameters constitute risk factors for consideration in the decision-making for AT in margin-negative stage IB and IIA NSCLC.
Clinical relevance statement
In this study, TLR from FDG PET/CT was an independent prognostic factor in stage IB-IIA non-small cell cancer patients. Although additional validation studies are warranted, TLR has the potential to be used to determine the need for adjuvant therapy.
Key Points
• High TLR is an independent poor prognostic factor in stage IB-IIA NSCLC.
• Adjuvant treatment should be considered in patients with high TLR following complete tumor resection.



Similar content being viewed by others
Abbreviations
- [18F]FDG PET/CT:
-
Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography
- AJCC:
-
American Joint Committee on Cancer
- AT:
-
Adjuvant treatment
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- EGFR:
-
Epidermal growth factor receptor
- ESMO:
-
European Society for Medical Oncology
- HR:
-
Hazard ratio
- LDH:
-
Lactate dehydrogenase
- LN:
-
Lymph node
- MD:
-
Moderately differentiated
- MTV:
-
Metabolic tumor volume
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- PD:
-
Poorly differentiated
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- SUVmax:
-
Maximum standardized uptake value
- TLG:
-
Total lesion glycolysis
- TLR:
-
Tumor-to-liver standardized uptake value ratio
- VPI:
-
Visceral pleural invasion
- WD:
-
Well differentiated
References
Postmus PE, Kerr KM, Oudkerk M et al (2017) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv1-iv21
National Comprehensive Cancer Network Non-Small Cell Lung Cancer (Version3.2022). Available via https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 3 June 2022
Zuo Z, Zhang G, Song P et al (2021) Survival nomogram for stage IB non-small-cell lung cancer patients, based on the SEER database and an external validation cohort. Ann Surg Oncol 28:3941–3950
Okiror L, Harling L, Toufektzian L et al (2018) Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J Thorac Cardiovasc Surg 156:785–793
Mollberg NM, Bennette C, Howell E, Backhus L, Devine B, Ferguson MK (2014) Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg 97:965–971
Peng T, Wightman SC, Ding L et al (2022) Lobectomy offers improved survival outcomes relative to segmentectomy for >2 but ≤4 cm non-small cell lung cancer tumors. JTCVS Open 10:356–367
Cao J, Yuan P, Wang Y et al (2018) Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg 105:1483–1491
Hou X, Yang MZ, Li JB et al (2022) Who are the real high-risk patients with pathological T2N0M0 non-small-cell lung cancer that can benefit from adjuvant chemotherapy? ESMO Open 7:100508
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT (2017) The eighth edition lung cancer stage classification. Chest 151:193–203
Lung cancer survival rates. Available via https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 30 June 2022
Strauss GM, Herndon JE 2nd, Maddaus MA et al (2008) Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26:5043–5051
Wong KM, Ding K, Li S et al (2017) A cost-effectiveness analysis of using the JBR.10-based 15-gene expression signature to guide adjuvant chemotherapy in early stage non-small-cell lung cancer. Clin Lung Cancer 18:e41–e47
Ng R, Hasan B, Mittmann N et al (2007) Economic analysis of NCIC CTG JBR.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer–a report of the Working Group on Economic Analysis, and the Lung Disease Site Group, National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:2256–2261
Liu J, Dong M, Sun X, Li W, Xing L, Yu J (2016) Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One 11:e0146195
Han EJ, Yang YJ, Park JC, Park SY, Choi WH, Kim SH (2015) Prognostic value of early response assessment using 18F-FDG PET/CT in chemotherapy-treated patients with non-small-cell lung cancer. Nucl Med Commun 36:1187–1194
YooIe R, Chung SK, Park HL et al (2014) Prognostic value of SUVmax and metabolic tumor volume on 18F-FDG PET/CT in early stage non-small cell lung cancer patients without LN metastasis. Biomed Mater Eng 24:3091–3103
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Jh O, Lodge MA, Wahl RL (2016) Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology 280:576–584
Huang J, Huang L, Zhou J et al (2017) Elevated tumor-to-liver uptake ratio (TLR) from (18)F-FDG-PET/CT predicts poor prognosis in stage IIA colorectal cancer following curative resection. Eur J Nucl Med Mol Imaging 44:1958–1968
Toledano MN, Vera P, Tilly H, Jardin F, Becker S (2019) Comparison of therapeutic evaluation criteria in FDG-PET/CT in patients with diffuse large-cell B-cell lymphoma: prognostic impact of tumor/liver ratio. PLoS One 14:e0211649
Wu C, Cui Y, Zhao Y et al (2020) Elevated tumor-to-liver standardized uptake value ratio (TLR) from preoperative (18)F-FDG PET/CT predicts poor prognosis of patients with clear cell renal cell carcinoma after nephrectomy. Eur J Radiol 131:109218
Park HL, Yoo IR, Boo SH et al (2019) Does FDG PET/CT have a role in determining adjuvant chemotherapy in surgical margin-negative stage IA non-small cell lung cancer patients? J Cancer Res Clin Oncol 145:1021–1026
Wu LL, Liu X, Jiang WM et al (2020) Stratification of patients with stage IB NSCLC based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual. Front Oncol 10:571
Consonni D, Pierobon M, Gail MH et al (2015) Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst 107:djv059
Zhang P, Duan J, Bai H et al (2021) Influence of adjuvant chemotherapy on survival for patients with stage IB and IIA non-small cell lung cancer. Thorac Cancer 12:30–39
Li X, Zhang C, Sun Z et al (2019) Propensity-matched analysis of adjuvant chemotherapy for completely resected stage IB non-small-cell lung cancer patients. Lung Cancer 133:75–82
Wang J, Wu N, Lv C, Yan S, Yang Y (2019) Should patients with stage IB non-small cell lung cancer receive adjuvant chemotherapy? A comparison of survival between the 8th and 7th editions of the AJCC TNM staging system for stage IB patients. J Cancer Res Clin Oncol 145:463–469
Horn L, Sandler AB, Putnam JB Jr, Johnson DH (2007) The rationale for adjuvant chemotherapy in stage I non-small cell lung cancer. J Thorac Oncol 2:377–383
Xie J, Zhang X, Hu S et al (2020) Effects of adjuvant chemotherapy on survival of patients with stage IB non-small cell lung cancer with visceral pleural invasion. J Cancer Res Clin Oncol 146:2231–2239
Douillard JY, Rosell R, De Lena M et al (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 7:719–727
Amin MB, Greene FL, Edge SB et al (2017) The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67:93–99
Hamanaka R, Yokose T, Sakuma Y et al (2015) Prognostic impact of vascular invasion and standardization of its evaluation in stage I non-small cell lung cancer. Diagn Pathol 10:17
Neri S, Yoshida J, Ishii G et al (2014) Prognostic impact of microscopic vessel invasion and visceral pleural invasion in non-small cell lung cancer: a retrospective analysis of 2657 patients. Ann Surg 260:383–388
Yang HC, Kim HR, Jheon S et al (2015) Recurrence risk-scoring model for Stage I Adenocarcinoma of the Lung. Ann Surg Oncol 22:4089–4097
Saji H, Okada M, Tsuboi M et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399:1607–1617
Su M, Li Y, Li F, Li L, Tian R (2014) Risk factors for N2 metastasis in patients with non-small-cell lung cancer: multivariate analyses of 18F-FDG PET/CT data. Nucl Med Commun 35:916–921
Yoo SW, Kim J, Chong A et al (2012) Metabolic tumor volume measured by F-18 FDG PET/CT can further stratify the prognosis of patients with stage IV non-small cell lung cancer. Nucl Med Mol Imaging 46:286–293
Qiu X, Liang H, Zhong W et al (2021) Prognostic impact of maximum standardized uptake value on (18) F-FDG PET/CT imaging of the primary lung lesion on survival in advanced non-small cell lung cancer: a retrospective study. Thorac Cancer 12:845–853
Adams MC, Turkington TG, Wilson JM, Wong TZ (2010) A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 195:310–320
Laffon E, Adhoute X, de Clermont H, Marthan R (2011) Is liver SUV stable over time in 18F-FDG PET imaging? J Nucl Med Technol 39:258–263
Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE (2009) Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol 11:118–122
Zwezerijnen GJC, Eertink JJ, Ferrández MC et al (2023) Reproducibility of [18F]FDG PET/CT liver SUV as reference or normalisation factor. Eur J Nucl Med Mol Imaging 50:486–493
Lim CH, Moon SH, Cho YS, Choi JY, Lee KH, Hyun SH (2019) Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with combined hepatocellular-cholangiocarcinoma. Eur J Nucl Med Mol Imaging 46:1705–1712
Wang C, Zhao K, Hu S et al (2020) The PET-derived tumor-to-liver standard uptake ratio (SUV (TLR) ) is superior to tumor SUVmax in predicting tumor response and survival after chemoradiotherapy in patients with locally advanced esophageal cancer. Front Oncol 10:1630
Lee JW, Oh JK, Chung YA et al (2016) Prognostic significance of 18F-FDG uptake in hepatocellular carcinoma treated with transarterial chemoembolization or concurrent chemoradiotherapy: a Multicenter retrospective cohort study. J Nucl Med 57:509–516
Piccardo A, Puntoni M, Bertagna F et al (2014) 18F-FDG uptake as a prognostic variable in primary differentiated thyroid cancer incidentally detected by PET/CT: a multicentre study. Eur J Nucl Med Mol Imaging 41:1482–1491
Evangelista L, Cuocolo A, Pace L et al (2018) Performance of FDG-PET/CT in solitary pulmonary nodule based on pre-test likelihood of malignancy: results from the ITALIAN retrospective multicenter trial. Eur J Nucl Med Mol Imaging 45:1898–1907
Lin JJ, Cardarella S, Lydon CA et al (2016) Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol 11:556–565
Aggarwal C, Bubendorf L, Cooper WA et al (2021) Molecular testing in stage I-III non-small cell lung cancer: approaches and challenges. Lung Cancer 162:42–53
Brody R, Zhang Y, Ballas M et al (2017) PD-L1 expression in advanced NSCLC:insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 112:200–215
Acknowledgements
We would like to thank So Young Jeon from the Department of Biostatistics at the Catholic University Graduate School for her statistical expertise.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ie Ryung Yoo.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Department of Biostatistics at the Catholic University kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, H.L., Boo, S.H., Park, S.Y. et al. Prognostic value of TLR from FDG PET/CT in patients with margin-negative stage IB and IIA non-small cell lung cancer. Eur Radiol 33, 7274–7283 (2023). https://doi.org/10.1007/s00330-023-09641-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09641-w