Abstract
Objective
Automatic MR imaging segmentation of the prostate provides relevant clinical benefits for prostate cancer evaluation such as calculation of automated PSA density and other critical imaging biomarkers. Further, automated T2-weighted image segmentation of central-transition zone (CZ-TZ), peripheral zone (PZ), and seminal vesicle (SV) can help to evaluate clinically significant cancer following the PI-RADS v2.1 guidelines. Therefore, the main objective of this work was to develop a robust and reproducible CNN-based automatic prostate multi-regional segmentation model using an intercontinental cohort of prostate MRI.
Methods
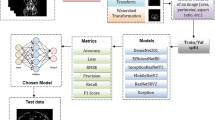
A heterogeneous database of 243 T2-weighted prostate studies from 7 countries and 10 machines of 3 different vendors, with the CZ-TZ, PZ, and SV regions manually delineated by two experienced radiologists (ground truth), was used to train (n = 123) and test (n = 120) a U-Net-based model with deep supervision using a cyclical learning rate. The performance of the model was evaluated by means of dice similarity coefficient (DSC), among others. Segmentation results with a DSC above 0.7 were considered accurate.
Results
The proposed method obtained a DSC of 0.88 ± 0.01, 0.85 ± 0.02, 0.72 ± 0.02, and 0.72 ± 0.02 for the prostate gland, CZ-TZ, PZ, and SV respectively in the 120 studies of the test set when comparing the predicted segmentations with the ground truth. No statistically significant differences were found in the results obtained between manufacturers or continents.
Conclusion
Prostate multi-regional T2-weighted MR images automatic segmentation can be accurately achieved by U-Net like CNN, generalizable in a highly variable clinical environment with different equipment, acquisition configurations, and population.
Key Points
• Deep learning techniques allows the accurate segmentation of the prostate in three different regions on MR T2w images.
• Multi-centric database proved the generalization of the CNN model on different institutions across different continents.
• CNN models can be used to aid on the diagnosis and follow-up of patients with prostate cancer.






Similar content being viewed by others
Data Availability
For data access, please, contact the authors of the manuscript.
Abbreviations
- ANOVA:
-
Analysis of variance
- CADe:
-
Computer-assisted detection
- CLR:
-
Cyclical learning rate
- CNN:
-
Convolutional neural network
- CZ:
-
Central zone
- DL:
-
Deep learning
- DRE:
-
Digital rectal examination
- DSC:
-
Dice score coefficient
- MAD:
-
Mean absolute distance
- PCa:
-
Prostate cancer
- PG:
-
Prostate gland
- PSA:
-
Prostate-specitic antigen
- PSAD:
-
Prostate-specitic antigen density
- PZ:
-
Peripheral zone
- SV:
-
Seminal vesicles
- TRUS:
-
Transrectal ultrasound
- TZ:
-
Transition zone
- ΔV:
-
Volume difference
References
De Visschere P (2018) Improving the diagnosis of clinically significant prostate cancer with magnetic resonance imaging. J Belg Soc Radiol 102(1):22
Cui T, Kovell RC, Terlecki RP (2016) Is it time to abandon the digital rectal examination? Lessons from the PLCO Cancer Screening Trial and peer-reviewed literature. Curr Med Res Opin 32(10):1663–1669
Mayo Clinic (2019) PSA test. Mayo Clinic. Available via https://www.mayoclinic.org/tests-procedures/psa-test/about/pac-20384731. Accessed 19 Jan 2023
Brown LC, Ahmed HU, Faria R et al (2018) Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess 22(39):1–176
Das CJ, Razik A, Netaji A, Verma S (2020) Prostate MRI-TRUS fusion biopsy: a review of the state of the art procedure. Abdom Radiol (NY) 45(7):2176–2183
Dai Z, Carver E, Liu C et al (2020) Segmentation of the prostatic gland and the intraprostatic lesions on multiparametic magnetic resonance imaging using mask region-based convolutional neural networks. Adv Radiat Oncol 5(3):473–481
da Silva GLF, Diniz PS, Ferreira JL et al (2020) Superpixel-based deep convolutional neural networks and active contour model for automatic prostate segmentation on 3D MRI scans. Med Biol Eng Comput 58(9):1947–1964
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 69(1):41–49
Hötker AM, Mazaheri Y, Aras Ö (2016) Assessment of prostate cancer aggressiveness by use of the combination of quantitative DWI and dynamic contrast-enhanced MRI. AJR Am J Roentgenol 206(4):756–763
Sanz-Requena R, Martí-Bonmatí L, Pérez-Martínez R, García-Martí G (2016) Dynamic contrast-enhanced case-control analysis in 3T MRI of prostate cancer can help to characterize tumor aggressiveness. Eur J Radiol 85(11):2119–2126
Gillespie D, Kendrick C, Boon I (2020) Deep learning in magnetic resonance prostate segmentation: a review and a new perspective. ArXiv: 2011.07795
Bardis M, Houshyar R, Chantaduly C (2021) Segmentation of the prostate transition zone and peripheral zone on MR images with deep learning. Radiol Imaging Cancer 3(3)
Cheng R, Lay N, Roth HR (2019) Fully automated prostate whole gland and central gland segmentation on MRI using holistically nested networks with short connections. J Med Imaging (Bellingham) 6(2):024007
Litjens G, Toth R, van de Ven W et al (2014) Evaluation of prostate segmentation algorithms for MRI: the PROMISE12 challenge. Med Image Anal 18(2):359–373
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Ronneberger O, Fischer P, Brox T (2015) U-Net convolutional networks for biomedical image segmentation. ArXiv: 1505.04597v1
Bo QZ, Turkbey B, Choyke PL (2017) Deeply-supervised CNN for prostate segmentation. ArXiv: 1703.07523
Kingma DP, Ba J (2017) Adam: a method for stochastic optimization. ArXiv: 1412.6980
Perez L, Wang J (2017) The effectiveness of data augmentation in image classification using deep learning. ArXiv: 1712.04621
Smith LN (2017) Cyclical learning rates for training neural networks. ArXiv: 1506.01186
Srisha R, Khan A (2013) Morphological operations for image processing: understanding and its applications. Conference: National Conference on VLSI, Signal processing & Communications
Kim DW, Jang HY, Kim KW (2019) Deep learning in magnetic resonance prostate segmentation: a review and a new perspective. Korean J Radiol 20(3):405–410
Shahedi M, Cool DW, Romagnoli C et al (2014) Spatially varying accuracy and reproducibility of prostate segmentation in magnetic resonance images using manual and semiautomated methods. Med Phys 41(11):113503
Brembilla G, Dell’Oglio P, Stabile A et al (2020) Interreader variability in prostate MRI reporting using Prostate Imaging Reporting and Data System version 2.1. Eur Radiol 30(6):3383–3392
Zavala-Romero O, Breto AL, Xu IR et al (2020) Segmentation of prostate and prostate zones using deep learning: A multi-MRI vendor analysis. Strahlenther Onkol 196(10):932–942
Lee DK, Sung DJ, Kim CS et al (2020) Three-dimensional convolutional neural network for prostate MRI segmentation and comparison of prostate volume measurements by use of artificial neural network and ellipsoid formula. AJR Am J Roentgenol 214(6):1229–1238
Khan Z, Yahya N, Alsaih K, Ali SSA, Meriaudeau F (2020) Evaluation of deep neural networks for semantic segmentation of prostate in T2W MRI. Sensors (Basel) 20(11):3183
Funding
The authors declare that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Angel Alberich-Bayarri.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: QUIBIM SL.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jimenez-Pastor, A., Lopez-Gonzalez, R., Fos-Guarinos, B. et al. Automated prostate multi-regional segmentation in magnetic resonance using fully convolutional neural networks. Eur Radiol 33, 5087–5096 (2023). https://doi.org/10.1007/s00330-023-09410-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09410-9