Abstract
Objectives
To explore the risk factors for recurrence of arterial complications after pancreatectomy during the period of covered stent implantation and to provide some opinions on peri-stent implantation management.
Methods
Data on patients implanted with covered stents due to arterial complications after pancreatectomy between January 2017 and December 2021 were analyzed retrospectively. Technical success, clinical success, recurrence, and survival were evaluated to elucidate the practicability of covered stents. Wilson score, Random Forest, logistic regression, and Pearson’s chi-square test with bootstrap aggregation were performed for determining the perioperative risk factors for recurrence.
Results
Among all fifty-five patients, success stent implantation (technical success) was achieved 100%. Patients who were hemodynamically stabilized without further treatment for artery complications in situ (clinical success) accounted for 89.1%. Based on statistical analysis, pre-stent implantation pancreatic fistula was identified as a robust recurrence-related risk factor for preoperative assessment (p = 0.02, OR = 4.5, 95% CI [1.2, 16.9]; pbootstrap = 0.02). Post-stent implantation pancreatic fistula (p = 0.01, OR 4.5, 95% CI [1.4, 14.6]; pbootstrap < 0.05) and SMA branches or GDA stumps (p = 0.02, OR 3.4, 95% CI [1.1, 10.3]) were relevant to recurrence. The survival rate during hospitalization was 87.3%. All survivors were free from recurrence during the subsequent follow-up. Vasospasm and stent occlusion were observed as short-term and long-term complications, respectively.
Conclusion
A covered stent implantation is a feasible and effective treatment option for post-pancreatectomy arterial complications. Rigorous management of pancreatic fistula, timely detection of problems, sensible strategies during stent implantation, and reasonable anticoagulation therapy are necessary for a better prognosis.
Key Points
• A covered stent is feasible for various artery-related complications after pancreatectomy and has an ideal therapeutic effect.
• Pancreatic fistula during the perioperative period of the covered stent is an independent risk factor for recurrent arterial complications and SMA branches or GDA stumps are prone to be recurrent offending arteries.
• Rigorous management of pancreatic fistula, timely detection of problems, sensible strategies during stent implantation, and reasonable anticoagulation therapy are necessary for a better prognosis.
Similar content being viewed by others
Introduction
The pancreas, endowed with a strategic position in the human body, abuts several major organs and abdominal vessels, which makes a series of pancreatic surgeries technically challenging. Although postoperative mortality (POM) in high-volume hospital systems has significantly decreased due to the centralization of high-risk surgery, the rate of postoperative complications is still relatively high (40–60%) compared with that of other abdominal operations, yet one-third of postoperative deaths are anticipated to be avoidable through rigorous management of perioperative complications [1,2,3].
Severe complications of pancreatectomy include biliary infection, abdominal infection, pancreatic fistula, and artery-related adverse events (ARAEs) that can occur spontaneously or be secondary to pancreatic fistula and infection [4,5,6,7,8]. Among them, due to the rapid progress of ARAEs, timely and effective treatment is required.
In recent years, endovascular procedures have been progressively regarded as the prime methods against ARAEs with the advantages of minimal invasion, outstanding clinical outcomes, and fewer complications [6, 9, 10]. Selective coil embolization can block the bleeding site or ruptured pseudoaneurysm quickly, especially for the tortuous arterial branches. However, the fact that vascular ligation during pancreatic surgery declines the blood supply of adjacent organs makes it necessary for interventional radiologists to retain the main artery and its important branches in the treatment of postpancreatectomy ARAEs. Stent graft implantation, broadly applied to intravascular interventional treatment, clinches the dilemma between sealing the target lesions and keeping the blood supply of downstream organs [11].
Several studies have shown that stent grafts are feasible for reversing ARAEs after pancreatectomy [8, 11,12,13]. However, most of them only focus on one kind of ARAEs and provide no comprehensive perspective of perioperative management. Therefore, the purpose of this study was to evaluate the clinical value of stent implantation in post-pancreatectomy ARAEs and explore the potential risk factors during the peri-stent period. We sought to present versatile and effective applications of stent grafts in ARAEs and provide experience in peri-stent implantation management.
Materials and methods
Patients
This retrospective study was approved by our institutional review board. All patients who underwent covered stent implantation as a therapeutic regimen for post-pancreatectomy ARAEs between January 2017 and December 2021 were included (n = 55). Other patients were excluded based on the criteria in Fig. 1a.
Procedure
The decision to perform stent implantation was made by pancreatic surgeons and interventional radiologists during consultations in the interventional department. The entire clinical course was shown in Fig. 1b. Informed patient consent was obtained from conscious patients or the immediate family of unconscious patients.
The patient was placed in a supine position, and the femoral artery (or the brachial artery) was punctured by the modified Seldinger method under local anesthesia. After the placement of a 5F vascular sheath (Terumo) through the access site, an angiographic catheter (aortography: pig catheter [Cordis]; selective angiography: RH catheter [Terumo] or C2 catheter [Cordis]; guidewire: 0.035 in. [Terumo]) was introduced for angiographic confirmation of arterial injury. The aortography and selective angiography in the celiac axis, superior mesenteric artery, and inferior mesenteric artery were carried out for patients with clinical bleeding manifestations. However, patients with ARAEs found in follow-up were supposed to undergo the selective angiography in offending artery directly and the selective angiographies in other arteries were finished after covered stent implantation to exclude other problems. The diameter (1–2 mm larger than that of the offending vessel) and length of the diseased artery were measured to determine the stent size after identifying the lesion site and checking the feasibility of stent implantation. If patients got arteriospasm caused by shock or redistribution of blood flow, the stent size was determined by the actual preoperative CT evaluation to avoid type I endoleak. The stent was intended to cover the proximal and distal 1 cm of the vascular rupture and maintain the patency of other arteries and their branches as much as possible. Then, a 7-10F sheath (Terumo) was employed in the ipsilateral artery to introduce a guiding catheter (Mach1, Boston). A 0.014 in. microguidewire (PT2, Boston) and a microcatheter (STC18, Boston Scientific) were adopted to assist operators in passing the distal end of the targeted artery. Next, a 0.018 in. guidewire (V-18, Boston) was exchanged and a GORE® VIABAHN® covered stent system (W. L. Gore & Associates) was introduced and released in the exact position, which completely and tightly covered the troublesome artery segment. The length of the covered stent was 25 mm or 50 mm with a diameter between 5 and 8 mm. After the stent placement was completed, the fine expansion and adhesion of the stent were verified by additional angiography (Fig. 2). If there was an extravascular branch adjacent to the diseased vessel, coil embolization was performed on this branch to avoid type II endoleak.
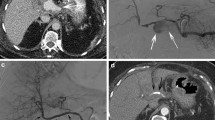
A 63-year-old woman after pancreaticoduodenectomy experienced bleeding in the drainage tube (so-called sentinel bleeding). Angiography showed the rough wall of the common hepatic artery and a distal narrowing of the common hepatic artery (a, black arrow). A hepatic pseudoaneurysm was also confirmed (a, black rectangle). Although there was no sign of contrast agent extravasation, hemorrhage, or ruptured pseudoaneurysm still could not be excluded. Therefore, a covered stent (Viabahn®, 6 × 50 mm) was implanted, and further angiography showed a fine stent position, normal luminal diameter, and no sign of pseudoaneurysm (b–d). The clinical manifestation of bleeding disappeared after stent implantation
Follow-up
All patients were given active support care and symptomatic therapy. Routine analysis of blood, liver, and kidney functions and coagulation indexes were monitored constantly. Abdominal CTA was reviewed at 1 month and 3 months after stent placement to evaluate stent patency. Follow-up was conducted through the analysis of electronic medical records and outpatient records.
Definitions
ARAEs were classified by modifying the ISGPS’s (International Study Group of Pancreatic Surgery) definition of post-pancreatectomy hemorrhage [14]. Technical success was defined as no angiographic signs of hemorrhage but the presence of significant patency of the diseased artery and its distal branches after stent placement. Clinical success was defined as hemodynamic stabilization without further treatment for ARAEs in situ after stent placement. Cases with recurrent ARAEs adjacent to the primary site were considered a clinical failure. Recurrent ARAEs was defined as recurrent arterial complications in primary or other arteries after stenting, which needed immediate re-intervention. The pancreatic fistula was classified according to the ISGPS’s definition and divided into pre-stent or post-stent implantation pancreatic fistula for analyzing the risk factor in different end points [15]. The first end point was defined when the covered stent implantation was accomplished. The second end point was defined when recurrent ARAEs took place.
Statistical analysis
Categorical variables were presented as numbers followed by percentages. Continuous variables were presented as the mean ± standard deviation. Due to the small sample size, Wilson score, Random Forest (number of trees: 500, random seed: 12), and the binary logistic regression with bootstrap aggregation were used to explore potential pre-stent implantation risk factors associated with recurrent ARAEs. Both leave-one-out cross-validation (LOOCV) and ROC curve with AUC value, Brier score, and C index were performed for model evaluation. Pearson’s chi-square test with or without bootstrap aggregation was used to compare the post-stent implantation clinical characteristics between the recurrence group and the no recurrence group in univariable analysis. p < 0.05 was defined as the level of statistical significance. The Wilson score was based on the lower limit of the Wilson score interval and applied for preliminary evaluation of the pre-stent risk factors (α = 0.05, Ζ ≈ 2). Random Forest model, LOOCV, and ROC curve with AUC value, Brier score, and C index were performed by using R (version 4.1.0) and R studio (version 1.1.463). The binary logistic regression and Pearson’s chi-square test with or without bootstrap aggregation (N = 1000, random seed: 1234) were performed by using SPSS for windows (version 2.0, IBM). The importance ranking and stepwise analysis of Random Forest were drawn by using GraphPad Prism 8 (GraphPad Software).
Results
Patient characteristics (Table 1)
A total of fifty-five patients (mean age, 63 years ± 11 [standard deviation]) were included in this study (Supplementary Material). The most common underlying disease was pancreatic ductal adenocarcinoma (n = 38). Most of the patients were surgically treated by pancreaticoduodenectomy (n = 36) and distal pancreatectomy with or without splenectomy (n = 12). In this study, 49.1% (n = 27) of the patients had suffered from pancreatic fistula (Grade B or higher) before covered stent implantation and 40% (n = 22) of the patients got new pancreatic fistula (Grade B or higher) after covered stent implantation or refractory pre-stent pancreatic fistula.
In this study, grade B ARAEs took place in 20% (n = 11) of the patients while 80% (n = 44) of the patients suffering from grade C ARAEs. Hemorrhage including ruptured pseudoaneurysm or dissecting aneurysm took place in 83.6% (n = 46) of the patients as the most common postsurgical ARAE. The primary clinical manifestation of hemorrhage was bleeding in the drainage tube or abdominal cavity (n = 46). Other ARAEs (n = 9) were mostly confirmed by radiological examinations as being asymptomatic except for one case with abdominal pain. The superior mesenteric artery (SMA) including its branches (n = 18) and the stump of the gastroduodenal artery (GDA) (n = 17) were the most two confirmed diseased vessels.
Technical and clinical success (Table 1)
Fifty-five patients accepted fifty-seven covered stent treatments, two of whom required second stent implantation for their refractory ARAEs identified by angiography. As shown in Fig. 3, 0.018 in. compliant system made covered stent get more adaptation in the treatment of ARAEs. Femoral artery access was achieved in fifty-two patients, while three patients were punctured transbrachially for SMA-related ARAEs due to the failed stent implantation through femoral access (Fig. 4).
A 71-year-old woman was confirmed bleeding in the junction of the main trunk of the superior mesenteric artery and branch of the jejunal artery (a). Coil embolization was used to block off the jejunal branch but another angiography showed contrast agent extravasation yet (b). This patient failed to receive a covered stent through femoral access. Therefore, the brachial artery was punctured and the stent (Viabahn®, 8 × 50 mm) was implanted successfully (c, location; d, release; e, angiography confirmed stent patency). Follow-up abdominal CTA reconstruction indicated an ideal treatment effect (f)
All cases were confirmed to show no contrast agent extravasation by angiography after fifty-seven stent implantations. The technical success rate of the endovascular stent treatment was 100%. However, immediate vasospasm of the proper hepatic artery at the far end of the stents was observed in two patients and was reversed successfully by trans-arterial injection of papaverine (3–6mg) or nitroglycerin (200–400μg). The clinical success rate of stent grafts applied to post-pancreatectomy ARAEs was 89.1% (n = 49). For six patients without clinical success, three patients confirmed recurrence in situ by angiography. One patient who underwent relaparotomy was found to have a new bleeding spot adjacent to the stump of the GDA. Two patients were unable to identify the exact bleeding spots. Although they were not confirmed recurrence in situ, these two cases were still considered a clinical failure from a conservative perspective (Table 2).
Recurrent ARAEs and potential risk factors
Recurrent ARAEs took place in 19 patients including 11 patients with identifiable re-hemorrhage, 6 patients with pseudoaneurysm formation, and 2 patients with unidentifiable re-hemorrhage (Table 3).
Based on results from the Wilson score at the first end point, grade of ARAEs (ΔS = 0.39), offending artery (ΔS = 0.26), pre-stent implantation pancreatic fistula (ΔS = 0.24), and age (ΔS = 0.23) were preliminarily identified as possible warning factors for recurrence (Table 4). To further explore the potential pre-stent risk factors, the Random Forest algorithm was adopted, and the importance ranking indicated that pre-stent pancreatic fistula, primary offending artery, and age were the most three important variables (Fig. 5a). The stepwise Random Forest was performed according to the result of importance ranking from high rank to low rank (Fig. 5b). The result showed that when the number of variables was 3, the out-of-bag error rate (OOB) was the lowest (OOB = 0.27). Therefore, the most three important variables in Random Forest were included in multivariable analysis, which showed that only pre-stent implantation grade B or higher pancreatic fistula (p = 0.02, OR = 4.5, 95% CI [1.2, 16.9]) was associated with recurrent ARAEs as a potential risk factor in pre-stent implantation assessment (Table 4). Due to the small sample size, bootstrap aggregation was performed during the logistic regression and the results indicated that both pre-stent grade B or higher pancreatic fistula (p = 0.02) and primary offending artery (p = 0.03) were significant. ROC curve was used to evaluate the model of logistic regression with bootstrap aggregation, which suggested an acceptable accuracy and efficiency (Fig. 5c). However, the kappa value of LOOCV was 0.36 and only 6 patients took place recurrence in situ. It reminded us that the primary offending artery in SMA or GDA had a warning effect but was not robust.
To evaluate the peri-stent implantation period comprehensively, a significant test with bootstrap aggregation was executed at the second end point. It should be mentioned that the sample size for analyzing the relationship between artery and recurrence is 91 because of a total sample of 110 arteries (Each patient has both two types of arteries, and those arteries are equally exposed in recurrence or not). The results reflected those patients with post-stent implantation grade B or higher pancreatic fistula were more liable to suffer from recurrent ARAEs (p = 0.01, OR 4.5, 95% CI [1.4, 14.6]; pbootstrap < 0.05). Compared with other arteries, SMA or gastroduodenal artery (GDA) had a higher rate of recurrent ARAEs (p = 0.02, OR 3.4, 95% CI [1.1, 10.3]) (Table 5). It may be related to the abundance of collateral vessels.
Survival
The survival rate during hospitalization was 87.3% (n = 48). Four patients underwent relaparotomy immediately after the rebleeding was confirmed. However, two of them passed away, expiring from infectious shock secondary to continuous rebleeding. One patient got continuous rebleeding even though another successfully covered stent in the common hepatic artery was implanted (Fig. 6). Five patients died due to cachexia and multiple organ failure. The patients surviving recurrent ARAEs underwent coil embolization or stent implantation and achieved both technical success and clinical success. There were no severe stent-related long-term complications, but stent thrombosis was found in one patient at 1-month follow-up, and in two patients at 3-month follow-up. All patients were free from recurrent ARAEs during the subsequent follow-up.
A 69-year-old man after pancreaticoduodenectomy experienced bleeding of the superior mesenteric artery and a covered stent (Viabahn®, 8 × 50 mm) was implanted (a, black arrow). However, abdominal CTA reconstruction after 12 days showed a hepatic pseudoaneurysm (b, black arrow) and a distal narrowing of the common hepatic artery (b, white arrow). Angiography confirmed the hepatic pseudoaneurysm (c, black arrow). Therefore, a covered stent (Viabahn®, 7 × 25 mm) was implanted, and further angiography showed a fine stent position and no sign of contrast agent extravasation (d, black arrow). Although two covered stents got technical success, this patient eventually died of infection shock
Discussion
Artery-related adverse events after pancreatectomy are mostly related to intraoperative injury of arteries, incomplete hemostasis of the surgical stump or anastomotic site, and vascular erosion caused by a pancreatic or biliary fistula and abdominal abscess [4, 16,17,18]. To date, there is still no clear consensus on the management of post-pancreatectomy ARAEs. Although it has been reported that coil embolization might incur postembolization syndrome, distal organ ischemia, infarction, or even necrosis, the risk is still relatively low in splenic, gastroduodenal, pancreatic, and mesenteric arteries due to collaterals formation [19,20,21]. This risk may increase in the proper hepatic artery, but it does not occur frequently after coil embolization. Stent implantation seems to be another satisfactory method against complex artery diseases with remarkable patency and has been applied in various vascular abnormalities [22, 23]. But it should be mentioned that covered stent implantation is still limited by the tortuosity of the visceral arteries in a minority of patients despite the adoption of the higher-compliant 0.018 in. guidewire and guide catheter has improved this problem to a certain extent. In this study, we collected cases of patients accepting covered stent implantation after pancreatectomy for various ARAEs. One hundred percent of the patients were treated with technical success, and 89.1% (n = 49) of the patients achieved complete control of the primary ARAEs, which demonstrated the broad applicability and excellent outcome of covered stents.
Pseudoaneurysm formation, chiefly resulting from pancreatic or biliary fistula and surgery-related abdominal infection, is a common complication after pancreatectomy, always with drainage bleeding as the primary symptom [14]. It was suggested that 34.5% (n = 19) of the patients in our research exhibited ruptured or unruptured pseudoaneurysms. All of them achieved technical success and 93.8% (n = 15) of the cases achieved clinical success after stent implantation. Intraoperative damage to the surrounding vessels or improper suturing of the stumps may result in the formation of dissecting aneurysms. These issues occurred in two patients in our study including a ruptured one. These kinds of “sentinel bleeding” often precede a potentially dangerous intra-abdominal hemorrhage in cases of ruptured (dissecting) pseudoaneurysm [24]. In consideration of the high risk of rupture, especially when complicated with pancreatic fistula, we recommend reasonable interventional treatment in patients with an aneurysm or dissecting aneurysm after pancreatectomy.
Pancreatic fistula after pancreatectomy should be treated immediately by drainage to avoid secondary infection and ARAEs in the operation area [4]. In this study, twenty-eight patients had suffered from grade B or higher pancreatic fistula when covered stents were planned to be placed and statistical analysis demonstrated that it was a potential risk factor for recurrent ARAEs in pre-stent implantation assessment. This study also indicated that post-stent grade B or higher pancreatic fistula was relevant to a high recurrent rate. Therefore, rapid control of pancreatic fistula is beneficial to the postoperative recovery of patients with ARAEs. Patients with grade B or higher pancreatic fistula should be closely monitored for hematologic indicators after stent implantation for a high risk of recurrent ARAEs.
Three patients with superior mesenteric ARAEs failed to implant stents from access to the femoral artery. Normally, the SMA forms an angle ranging from 38 to 65° when it comes off the aorta, which made it difficult for stents to be introduced into the lesion site transfemorally [25]. Therefore, obtuse angle access obtained from the brachial artery approach may be more suitable against SMA-related adverse events. However, transbrachial access requires a higher proficiency from interventional clinicians due to the risk of postoperative hematoma and nerve compression symptoms [26,27,28]. Operators should make quick and correct decision during the operation according to the actual situation. Further study is needed for a more optimized regimen to treat SMA complications.
In this study, low-molecular-weight heparin anticoagulant therapy (0.3 mL/3200IU S.C. Q12H) was generally started when the patient’s blood pressure and hemoglobin level became stable after stent implantation, and long-term oral aspirin (100 mg P.O. Q.D.) and clopidogrel (75 mg P.O. Q.D.) antiplatelet therapy were implemented in all surviving patients. But stent occlusion was still observed in three patients in follow-up CTAs. However, several studies have reported anticoagulant-related bleeding in patients with stent implantation [29, 30]. Therefore, individualized regimens should be explored and tailored.
The limitations of our research can be summarized as follows. First, this is a single-center, retrospective study. Second, most patients in this study suffered from malignant tumor, which may have an influence on clinical outcomes. Finally, the lethality of post-pancreatectomy ARAEs makes it difficult to set up a traditional control group for comparing covered stents with other strategies. Therefore, there was only a comparison with the same method from an ethical perspective.
In conclusion, our results demonstrate that a covered stent is a safe and effective interventional technique for dealing with postpancreatectomy ARAEs while maintaining the patency of target vessels. As the basic clinical status of patients after pancreatic surgery is poor, seasoned interventional clinicians and multidisciplinary perioperative management are essential for patients to acquire long-term survival with high quality of life.
Abbreviations
- ARAEs:
-
Artery-related adverse events
References
Sheetz KH, Dimick JB, Nathan H (2019) Centralization of high-risk cancer surgery within existing hospital systems. J Clin Oncol 37:3234–3242
Smits FJ, Verweij ME, Daamen LA et al (2022) Impact of complications after pancreatoduodenectomy on mortality, organ failure, hospital stay, and readmission: analysis of a nationwide audit. Ann Surg 275:e222–e228
Beugniez C, Sauvanet A, Sulpice L et al (2021) Root-cause analysis of mortality after pancreatic resection (CARE Study): a multicenter cohort study. Ann Surg 274:789–796
Smits FJ, van Santvoort HC, Besselink MG et al (2017) Management of severe pancreatic fistula after pancreatoduodenectomy. JAMA Surg 152:540–548
Suragul W, Rungsakulkij N, Vassanasiri W et al (2020) Predictors of surgical site infection after pancreaticoduodenectomy. BMC Gastroenterol 20:201
Yekebas EF, Wolfram L, Cataldegirmen G et al (2007) Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 246:269–280
Cui L, Kong L, Bai YH et al (2020) Covered stent placement for hepatic artery pseudoaneurysm. Abdom Radiol (NY) 45:3337–3341
You Y, Choi SH, Choi DW et al (2019) Long-term clinical outcomes after endovascular management of ruptured pseudoaneurysm in patients undergoing pancreaticoduodenectomy. Ann Surg Treat Res 96:237–249
Oderich GS, Tenorio ER, Mendes BC et al (2021) Midterm outcomes of a prospective, nonrandomized study to evaluate endovascular repair of complex aortic aneurysms using fenestrated-branched endografts. Ann Surg 274:491–499
Illuminati G, Hostalrich A, Pasqua R, Nardi P, Chaufour X, Ricco JB (2021) Outcomes after open and endovascular repair of non-ruptured true pancreaticoduodenal and gastroduodenal artery aneurysms associated with coeliac artery compression: a multicentre retrospective study. Eur J Vasc Endovasc Surg 61:945–953
Schaarschmidt BM, Boos J, Buchbender C et al (2018) Heparin-bonded stent graft treatment for major visceral arterial injury after upper abdominal surgery. Eur Radiol 28:3221–3227
Hassold N, Wolfschmidt F, Dierks A, Klein I, Bley T, Kickuth R (2016) Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J Vasc Surg 64:1373–1383
Muglia R, Lanza E, Poretti D et al (2020) Emergency endovascular treatments for delayed hemorrhage after pancreaticobiliary surgery: indications, outcomes, and follow-up of a retrospective cohort. Abdom Radiol (NY) 45:2593–2602
Wente MN, Veit JA, Bassi C et al (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula. Surgery 161(3):584-591
Landen S, Ursaru D, Delugeau V, Landen C (2017) How to deal with hepatic artery injury during pancreaticoduodenectomy. A systematic review. J Visc Surg 154:261–268
Ratnayake CBB, Wells C, Hammond J, French JJ, Windsor JA, Pandanaboyana S (2019) Network meta-analysis comparing techniques and outcomes of stump closure after distal pancreatectomy. Br J Surg 106:1580–1589
Matsumoto I, Kamei K, Satoi S et al (2021) Conversion to open laparotomy during laparoscopic distal pancreatectomy: lessons from a single-center experience in 70 consecutive patients. Surg Today 51:70–78
Swierz MJ, Storman D, Riemsma RP et al (2020) Transarterial (chemo)embolisation versus no intervention or placebo for liver metastases. Cochrane Database Syst Rev 3:CD009498
Cho JY, Han HS, Choi Y et al (2017) Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg 152:386–392
Ishikawa H, Ohbe H, Omachi N, Morita K, Yasunaga H (2021) Spinal cord infarction after bronchial artery embolization for hemoptysis: a nationwide observational study in Japan. Radiology 298:673–679
Li X, Li W, Dai X et al (2021) Thoracic endovascular repair for aortic arch pathologies with surgeon modified fenestrated stent grafts: a multicentre retrospective study. Eur J Vasc Endovasc Surg. 62:758–766
Leo E, Molinari ACL, Ferraresi M, Rossi G (2021) Short term outcomes of Distal Extended EndoVascular Aortic Repair (DEEVAR) petticoat in acute and subacute complicated type B Aortic Dissection. Eur J Vasc Endovasc Surg 62:569–574
Arvieux C, Frandon J, Tidadini F et al (2020) Effect of prophylactic embolization on patients with blunt trauma at high risk of splenectomy: a randomized clinical trial. JAMA Surg 155:1102–1111
Pottorf BJ, Husain FA, Hollis HW, Lin E (2014) Laparoscopic management of duodenal obstruction resulting from superior mesenteric artery syndrome. JAMA Surg 149:1319–1322
Treitl KM, König C, Reiser MF, Treitl M (2015) Complications of transbrachial arterial access for peripheral endovascular interventions. J Endovasc Ther 22:63–70
Kret MR, Dalman RL, Kalish J, Mell M (2016) Arterial cutdown reduces complications after brachial access for peripheral vascular intervention. J Vasc Surg 64:149–154
Preisner F, Bendszus M, Schwarz D (2020) Visualization of direct median nerve damage following transbrachial arterial access. JACC Cardiovasc Interv 13:1265–1266
Zhang X, Huang J, Peng Z, Lu X, Yang X, Ye K (2021) Comparing safety and efficacy of rivaroxaban with warfarin for patients after successful stent placement for chronic iliofemoral occlusion: a retrospective single institution study. Eur J Vasc Endovasc Surg 61(3):484–489
Leggio M, Fusco A, Severi P et al (2018) Antithrombotic therapy after percutaneous coronary intervention in atrial fibrillation: the triple trouble. Drugs 78:1309–1319
Acknowledgements
We thank the companions from the Department of Interventional Radiology, Luwan Branch of Ruijin Hospital for their encouragement and support during the coronavirus epidemic period.
Funding
This work was supported by Shanghai Key Specialty Construction Project (ZK2019A02) and Shanghai Municipal Key Clinical Specialty (shslczdzk07002 and shslczdzk06002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhongmin Wang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 15 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Huang, W., Liu, Q. et al. Covered stent treatment for arterial complications after pancreatic surgery: risk assessment for recurrence and peri-stent implantation management. Eur Radiol 33, 1779–1791 (2023). https://doi.org/10.1007/s00330-022-09134-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09134-2