Abstract
Objectives
To investigate the efficacy and safety of dicycloplatin as chemotherapeutic regimen in transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma (HCC).
Methods
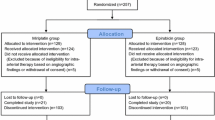
In this randomized, open-label, phase II trial, patients with unresectable HCC who were TACE treatment–naïve or experienced recurrence after surgical resection or ablation were enrolled at 7 centers in China from March 2019 to November 2019. Participants were randomly assigned (1:1:1) to receive TACE with chemotherapeutic regimen of dicycloplatin alone (group A1), dicycloplatin plus epirubicin (group A2), or epirubicin alone (group B). The primary endpoint was objective response rate (ORR). The secondary endpoints included disease control rate (DCR), duration of response (DOR), progression-free survival (PFS), and safety.
Results
The ORR at 6 months in group A1 (n = 22) was significantly better than that in group B (p = 0.093; 90% confidence interval [CI], 1.03–9.45). The DCR in group A1 was significantly higher than that in group B (p = 0.045; 90% CI, 1.29–12.88). There was no significant difference in DOR among the groups (p = 0.271). The median PFS were 6.00 and 3.05 months in groups A2 (n = 25) and B (n = 24), respectively (p = 0.061). Grade 3 or worse adverse events were similar among groups in the safety population (p = 0.173).
Conclusion
TACE with dicycloplatin was comparably safe and well tolerable as epirubicin alone in patients with unresectable HCC. Compared with epirubicin alone, significant improvement in ORR and DCR when dicycloplatin was applied, as well as prolonged PFS when dicycloplatin plus epirubicin was applied, was generated.
Key Points
• To our knowledge, this is the first multicenter randomized trial to assess the efficacy and safety of TACE with dicycloplatin in patients with unresectable HCC.
• This phase II trial showed that TACE with dicycloplatin alone or plus epirubicin was comparably safe and well tolerable as epirubicin alone.
• Significant improvements in ORR, DCR when dicycloplatin was applied, and prolonged PFS when dicycloplatin plus epirubicin was applied were recorded compared with epirubicin alone.



Similar content being viewed by others
Abbreviations
- ALT:
-
Glutamic pyruvic transaminase
- AST:
-
Glutamic oxaloacetic transaminase
- CR:
-
Complete response
- DCR:
-
Disease control rate
- DOR:
-
Duration of response
- HCC:
-
Hepatocellular carcinoma
- IQR:
-
Interquartile range
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- ORR:
-
Objective response rate
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PS:
-
Performance status
- SD:
-
Stable disease
- TACE:
-
Transarterial chemoembolization
References
Llovet JM, Kelley RK, Villanueva A et al (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7:6
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171
Park JW, Chen M, Colombo M et al (2015) Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 35:2155–2166
Zhou J, Sun H, Wang Z et al (2020) Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 9:682–720
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines:Management of hepatocellular carcinoma. J Hepatol 69:182–236
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11:317–370
Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E (2012) Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol 35:765–774
Chen LT, Martinelli E, Cheng AL et al (2020) Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 31:334–351
Guo T, Wu P, Liu P et al (2018) Identifying the best anticancer agent combination in TACE for HCC patients: a network meta-analysis. J Cancer 9:2640–2649
Ikeda M, Kudo M, Aikata H et al (2018) Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J Gastroenterol 53:281–290
Harper BW, Krause-Heuer AM, Grant MP, Manohar M, Garbutcheon-Singh KB, Aldrich-Wright JR (2010) Advances in platinum chemotherapeutics. Chemistry 16:7064–7077
Zalba S, Garrido MJ (2013) Liposomes, a promising strategy for clinical application of platinum derivatives. Expert Opin Drug Deliv 10:829–844
Wheate NJ, Walker S, Craig GE, Oun R (2010) The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 39:8113–8127
Goncalves MS, Silveira AF, Teixeira AR, Hyppolito MA (2013) Mechanisms of cisplatin ototoxicity: theoretical review. J Laryngol Otol 127:536–541
Qaddoumi I, Bass JK, Wu J et al (2012) Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol 30:1034–1041
Yu JJ, Yang X, Song Q, Mueller MD, Remick SC (2014) Dicycloplatin, a novel platinum analog in chemotherapy: synthesis of Chinese pre-clinical and clinical profile and emerging mechanistic studies. Anticancer Res 34:455–463
Yu JJ, Hogan T, Morley C et al (2019) Adverse effects profile of dicycloplatin (DCP) offers chemotherapeutic advantage over cisplatin and carboplatin. Anticancer Res 39:4455–4462
Yang X, Zheng J, Song Q et al (2015) Determination methods for the anticancer drug dicycloplatin, a supramolecule assembled through hydrogen bonding. Analyst 140:2704–2712
Li GQ, Chen XG, Wu XP et al (2012) Effect of dicycloplatin, a novel platinum chemotherapeutical drug, on inhibiting cell growth and inducing cell apoptosis. PLoS One 7:e48994
Liu KJ, Guan ZZ, Liang Y et al (2014) A double-blind, randomized phase II study of dicycloplatin plus paclitaxel versus carboplatin plus paclitaxel as first-line therapy for patients with advanced non-small-cell lung cancers. Arch Med Sci 10:717–724
Zhao D, Zhang Y, Xu C et al (2012) Pharmacokinetics, tissue distribution, and plasma protein binding study of platinum originating from dicycloplatin, a novel antitumor supramolecule, in rats and dogs by ICP-MS. Biol Trace Elem Res 148:203–208
Lu J, Zhong BY, Zhu HD, Guo JH, Teng GJ (2019) Embolotherapy of unresectable hepatocellular carcinoma: eastern perspective. Chin Clin Oncol 8:60
Sahara S, Kawai N, Sato M et al (2012) Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti-cancer drugs (epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 35:1363–1371
Sahara S, Kawai N, Sato M et al (2010) Prospective comparison of transcatheter arterial chemoembolization with lipiodol-epirubicin and lipiodol-cisplatin for treatment of recurrent hepatocellular carcinoma. Jpn J Radiol 28:362–368
Park JW, Amarapurkar D, Chao Y et al (2013) Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int 33:327–337
Yamanaka K, Hatano E, Narita M et al (2011) Comparative study of cisplatin and epirubicin in transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Res 41:303–309
Homma H, Mezawa S, Doi T et al (2004) A comparative randomized trial of intermittent intrahepatic arterial carboplatin- versus doxorubicin-lipiodol emulsion in advanced hepatocellular carcinoma (stage IV). Hepatogastroenterology 51:1135–1139
Apps MG, Choi EH, Wheate NJ (2015) The state-of-play and future of platinum drugs. Endocr Relat Cancer 22:R219–R233
Zhao M, Xiang P, Jiang H (2018) Transarterial chemoembolization (TACE) with platinum versus anthracyclines for hepatocellular carcinoma: a meta-analysis. Int J Surg 53:151–158
Li S, Huang H, Liao H et al (2013) Phase I clinical trial of the novel platin complex dicycloplatin: clinical and pharmacokinetic results. Int J Clin Pharmacol Ther 51:96–105
Acknowledgements
We thank the patients and their families for participating in this trial.
Funding
The funders of the study (Beijing Suopuxingda Pharmaceutical Research Co., Ltd.) had no role in study protocol design, data analysis and interpretation, or writing of the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
Written informed consent was obtained from all trial participants included in the study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Interventional
• Performed at seven institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Zhu, HD., Li, X., Ji, JS. et al. TACE with dicycloplatin in patients with unresectable hepatocellular carcinoma: a multicenter randomized phase II trial. Eur Radiol 32, 7335–7343 (2022). https://doi.org/10.1007/s00330-022-08848-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08848-7