Abstract
Objectives
This study aimed to evaluate the feasibility of automatic Stanford classification of classic aortic dissection (AD) using a 2-step hierarchical neural network.
Methods
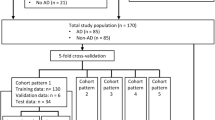
Between 2015 and 2019, 130 arterial phase series (57 type A, 43 type B, and 30 negative cases) in aortic CTA were collected for the training and validation. A 2-step hierarchical model was built including the first step detecting AD and the second step predicting the probability (0–1) of Stanford types. The model’s performance was evaluated with an off-line prospective test in 2020. The sensitivity and specificity for Stanford type A, type B, and no AD (Sens A, B, N and Spec A, B, N, respectively) and Cohen’s kappa were reported.
Results
Of 298 cases (22 with type A, 29 with type B, and 247 without AD) in the off-line prospective test, the Sens A, Sens B, and Sens N were 95.45% (95% confidence interval [CI], 77.16–99.88%), 79.31% (95% CI, 60.28–92.01%), and 93.52% (95% CI, 89.69–96.25%), respectively. The Spec A, Spec B, and Spec N were 98.55% (95% CI, 96.33–99.60%), 94.05% (95% CI, 90.52–96.56%), and 94.12% (95% CI, 83.76–98.77%), respectively. The classification rate achieved 92.28% (95% CI, 88.64–95.04%). The Cohen’s kappa was 0.766 (95% CI, 0.68–0.85; p < 0.001).
Conclusions
Stanford classification of classic AD can be determined by a 2-step hierarchical neural network with high sensitivity and specificity of type A and high specificity in type B and no AD.
Key Points
• The Stanford classification for aortic dissection is widely adopted and divides it into Stanford type A and type B based on the ascending thoracic aorta dissected or not.
• The 2-step hierarchical neural network for Stanford classification of classic aortic dissection achieved high sensitivity (95.45%) and specificity (98.55%) of type A and high specificity in type B and no aortic dissection (94.05% and 94.12%, respectively) in 298 test cases.
• The 2-step hierarchical neural network demonstrated moderate agreement (Cohen’s kappa: 0.766, p < 0.001) with cardiovascular radiologists in detection and Stanford classification of classic aortic dissection in 298 test cases.




Similar content being viewed by others
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- AD:
-
Aortic dissection
- CI:
-
Confidence interval
- CNN:
-
Convolutional neural network
- TEVAR:
-
Thoracic endovascular aortic repair
References
Bossone E, LaBounty TM, Eagle KA (2018) Acute aortic syndromes: diagnosis and management, an update. Eur Heart J 39:739–749d
Yu HY, Chen YS, Huang SC, Wang SS, Lin FY (2004) Late outcome of patients with aortic dissection: study of a national database. Eur J Cardiothorac Surg 25:683–690
Mészáros I, Mórocz J, Szlávi J et al (2000) Epidemiology and clinicopathology of aortic dissection. Chest 117:1271–1278
Clouse WD, Hallett JWJ, Schaff HV et al (2004) Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 79:176–180
McMahon MA, Squirrell CA (2010) Multidetector CT of aortic dissection: a pictorial review. Radiographics 30:445–460
Dohnert W (2005) Cardiovascular disorders: aortic dissection, 5th edn. Lippincott Williams & Wilkins, Philadelphia
Karmy-Jones R, Aldea G, Boyle EM Jr (2000) The continuing evolution in the management of thoracic aortic dissection. Chest 117:1221–1223
Tsai TT, Nienaber CA, Eagle KA (2005) Acute aortic syndromes. Circulation 112:3802–3813
Yasaka K, Abe O (2018) Deep learning and artificial intelligence in radiology: current applications and future directions. PLoS Med 15:e1002707
Dehghan E, Wang H, Syeda-Mahmood T (2017) Automatic detection of aortic dissection in contrast-enhanced CT. 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), pp 557–560
Harris RJ, Kim S, Lohr J et al (2019) Classification of aortic dissection and rupture on post-contrast CT images using a convolutional neural network. J Digit Imaging 32:939–946
Krissian K, Carreira JM, Esclarin J, Maynar M (2014) Semi-automatic segmentation and detection of aorta dissection wall in MDCT angiography. Med Image Anal 18:83–102
Duan X, Shi M, Wang J, Zhao H, Chen D (2016) Segmentation of the aortic dissection from CT images based on spatial continuity prior model. 2016 8th International Conference on Information Technology in Medicine and Education (ITME):275–280
Yellapragada MS, Xie Y, Graf B, Richmond D, Krishnan A, Sitek A (2020) Deep learning based detection of acute aortic syndrome in contrast CT images. 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI). IEEE, pp 1474–1477
Oktay O, Schlemper J, Folgoc LL et al (2018) Attention u-net: Learning where to look for the pancreas. arXiv preprint arXiv:180403999
Panchal A, Trivedi H, Rajput M, Trivedi D (2020) Activity recognition using temporal features and deep bottleneck 3d-resnext. Springer Singapore, Singapore, pp 889–904
Zou KH, Warfield SK, Bharatha A et al (2004) Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol 11:178–189
Beleites C, Neugebauer U, Bocklitz T, Krafft C, Popp J (2013) Sample size planning for classification models. Anal Chim Acta 760:25–33
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282
Tan Y, Tan L, Xiang X, Tang H, Qin J, Pan W (2020) Automatic detection of aortic dissection based on morphology and deep learning. Comput Mater Contin 62:1201–1215
Cheng J, Tian S, Yu L, Ma X, Xing Y (2020) A deep learning algorithm using contrast-enhanced computed tomography (CT) images for segmentation and rapid automatic detection of aortic dissection. Biomed Signal Process Control 62:102145
Hata A, Yanagawa M, Yamagata K et al (2021) Deep learning algorithm for detection of aortic dissection on non-contrast-enhanced CT. Eur Radiol 31:1151–1159
Wong T-T (2015) Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recogn 48:2839–2846
Hussain Z, Gimenez F, Yi D, Rubin D (2017) Differential data augmentation techniques for medical imaging classification tasks. AMIA Annu Symp Proc 2017:979–984
Hiratzka LF, Bakris GL, Beckman JA et al (2010) 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 121:e266–e369
Vardhanabhuti V, Nicol E, Morgan-Hughes G et al (2016) Recommendations for accurate CT diagnosis of suspected acute aortic syndrome (AAS)–on behalf of the British Society of Cardiovascular Imaging (BSCI)/British Society of Cardiovascular CT (BSCCT). Br J Radiol 89:20150705
Adler P, Falk C, Friedler SA et al (2018) Auditing black-box models for indirect influence. Knowl Inf Syst 54:95–122
Alufaisan Y, Kantarcioglu M, Zhou Y (2016) Detecting discrimination in a black-box classifier. 2016 IEEE 2nd International Conference on Collaboration and Internet Computing (CIC). IEEE, pp 329–338
Datta A, Sen S, Zick Y (2016) Algorithmic transparency via quantitative input influence: theory and experiments with learning systems. 2016 IEEE Symposium on Security and Privacy (SP), pp 598–617
Acknowledgements
The authors would like to thank Convergence CT for assistance with English editing.
Funding
This study has received funding from the Ministry of Science and Technology of Taiwan (Grants 109–2634-F-006–023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chien-Kuo Wang.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Po-Tsun, Paul, Kuo: an employee in the AI Research Centre, Advantech Company.
Authors who are not employees of or consultants for Advantech Company had control of the inclusion of any data and information that might present a conflict of interest for the author who is an employee of that industry.
The other authors have no conflict of interest to disclose.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, LT., Tsai, YS., Liou, CF. et al. Automated Stanford classification of aortic dissection using a 2-step hierarchical neural network at computed tomography angiography. Eur Radiol 32, 2277–2285 (2022). https://doi.org/10.1007/s00330-021-08370-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08370-2